How to synthesize Magnesium carbonate?
Introduction
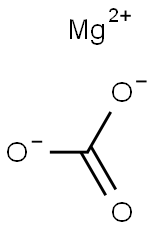
Magnesium carbonate, or magnesite, is a magnesium salt with formula MgCO3. It is a magnesium salt, a carbonate salt, a one-carbon compound and an organic magnesium salt. Its hydrated forms, particularly the di-, tri-, and tetrahydrates, occur as minerals. It is a common over-the-counter remedy for heartburn and upset stomach caused by the overproduction of acid in the stomach[1].

Uses
Because of its water-insoluble, hygroscopic properties, MgCO3 was first added to salt in 1911 to make it flow more freely. Magnesium carbonate, often called “chalk”, is used as a drying agent for hands in gymnastics, weight lifting and rock climbing. Magnesium carbonate is also used in taxidermy to whiten skulls. It can be mixed with hydrogen peroxide to create a paste and then spread on the skull to give it a white finish. Basic magnesium carbonate is used as clay in face masks. It has mild astringent properties and helps smooth and soften skin. It is recommended for use on normal to dry skin.
Magnesium carbonate is one of many salts of magnesium used clinically. The carbonate and other salts, such as trisilicate, citrate, oxide and sulfate, are used widely for the relief of gastrointestinal symptoms of dyspepsia, heartburn, gastroesophageal reflux disease, and constipation by acting as antacids and laxatives. Although the carbonate is only used as an antacid or laxative, some salts may also have other uses, as indicated in records for the individual salts. The sulfate salt, in particular, has many additional medical uses. Like several other magnesium salts, the carbonate is rarely given alone and is usually combined with other antacids. The anion attached to the magnesium can affect the pharmacokinetics and the delivery of the elemental magnesium to the target site.
Synthesis
A preparation method for magnesium carbonate comprises the following steps:
Magnesium sulfate particle is joined to sodium carbonate solution by step 1);
Step 2) Obtain magnesium carbonate precipitation and metabisulfite solution after reaction for some time;
From the solution, filtering separation is out by magnesium carbonate precipitation for step 3);
Step 4) Magnesium carbonate distilled water is washed 2-4 times, then dried and processed with the baker, obtaining pure magnesium carbonate.
Further, in described step 1), magnesium sulfate chooses food grade magnesium sulfate, sodium carbonate chooses food grade sodium carbonate, and the mass ratio of magnesium sulfate and sodium carbonate is 1:1.
Described step 2) The temperature of the reaction gets a normal temperature, and the reaction time is 30-45min.
Described step 4) bake out temperature is 95-100 ℃, and drying time is 1-1.5h. The magnesium carbonate described in step 4) is obtained is food-grade magnesium carbonate[2].
In specific:
The food grade magnesium sulfate particle getting 0.5mol joins in the food grade sodium carbonate solution of 0.5mol/L, magnesium carbonate precipitation and metabisulfite solution is obtained after reacting 5h under normal temperature, by magnesium carbonate precipitation, from solution, filtering separation is out, by magnesium carbonate distilled water wash 2 times, at 50 ℃, dry 1h with the baker, obtain pure magnesium carbonate.
References
[1] US2765212A - Preparation of magnesium carbonate - Google Patents https://patents.google.com/patent/US2765212A/en
[2] CN104477951A - Preparation method of magnesium carbonate - Google Patents https://patents.google.com/patent/CN104477951A/en
You may like
Related articles And Qustion
Lastest Price from Magnesium carbonate manufacturers

US $1200.00-1100.00/ton2025-09-23
- CAS:
- 546-93-0
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
US $3.00-9.00/KG2025-06-19
- CAS:
- 546-93-0
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available