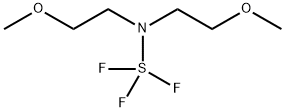
Bis(2-Methoxyethyl)aminosulfur Trifluoride: Versatility in Fluorination Reactions
General Description
Bis(2-methoxyethyl)aminosulfur trifluoride (abbreviated as Deoxo-FluorTM) is a broad-spectrum deoxyfluorinating agent with enhanced thermal stability, which is mostly used in organic chemical reactions for the preparation of other heterocyclic compounds. Deoxo-FluorTM is effective for the conversion of alcohols to alkyl fluorides, aldehydes/ketones to their corresponding difluorinated gemstones, and carboxylic acids to their trifluoromethyl derivatives, and is less thermally sensitive and broader in scope than traditional dialkylaminosulfur trifluoride (DAST) deoxidising fluorinating reagents.

Figure 1. Bis(2-methoxyethyl)aminosulfur trifluoride
Overview
Chemical Properties
Deoxo-FluorTM has the appearance of a colourless to yellow to orange transparent liquid and is moisture-sensitive and heat-sensitive and therefore needs to be stored under inert gas. It is a severe skin and eye irritant, corrosive to the skin and toxic if swallowed.
In addition to thermal instability, DAST and Deoxo-FluorTM have other drawbacks, especially in handling. They are fuming liquids that react rapidly with atmospheric moisture, even in non-humid environments, and react violently with water to produce large quantities of toxic and corrosive gases such as HF and SO2 These liquids also become discoloured with age and sometimes need to be re-distilled to be fit for purpose.
Advantages and Applications
Diethylsulfur trifluoride (DAST) and Deoxo-FluorTM are widely used as nucleophilic fluorination reagents for the synthesis of biologically active organic molecules. Compared with DAST, Deoxo-Fluor is superior in terms of thermal stability and reactivity. Differential scanning calorimetry (DSC) showed that the decomposition temperatures of DAST and Deoxo-FluorTM were the same at 140 °C, with the difference that DAST degraded faster with an enthalpy change ΔH of -1700 J/g, whereas the decomposition ΔH of Deoxo-FluorTM was smaller at -1100 J/g. This stability of Deoxo-FluorTM is essential to expand its range of applications, making it useful in both chemical research and industrial processes, contributing to the precise generation of fluorinated compounds. As a result, Deoxo-FluorTM is often the first choice for complex deoxyfluorination reactions to produce fluorinated intermediates necessary for further synthesis.
Applications in Organic Chemistry
Fluorination of Thiocarbonyl Compounds
Deoxo-FluorTM also plays an important role in the fluorination of thiocarbonyl compounds including thione, thioesters, thioamides, dithioesters and dithiocarbamates. The reagent has been shown to efficiently convert these different thiocarbonyl derivatives into the corresponding fluorinated gemstones in very high yields.
One-Pot Synthesis of Amides and Oxazolines
Deoxo-FluorTM is also widely used in one-pot synthesis of amides or oxazolines directly from carboxylic acids. A variety of free fatty acids are synthesised simultaneously in very high yields using 2-amino-2,2-dimethyl-1-propanol via one-step condensation or condensation-cyclisation reactions.2
Enhancing Synthetic Accessibility
The use of Deoxo-FluorTM significantly enhances the synthetic accessibility of complex fluorinated compounds and nitrogen-containing heterocycles in organic chemistry. Its role in the efficient conversion of thiocarbonyl derivatives and the straightforward one-pot synthesis demonstrates its importance as a fluorinating agent. The ability to facilitate these transformations with commendable yields opens new avenues for researchers looking to incorporate fluorine into organic molecules, given the increasing importance of fluorinated compounds in pharmaceuticals and agrochemicals.3
References:
[1] CYROUS O. KANGANI D E K. One-Pot Direct Synthesis of Amides or Oxazolines from Carboxylic Acids Using Deoxo-Fluor Reagent.[J]. ChemInform, 2006, 37 13. DOI:10.1002/chin.200613066.[2] GAURI S. LAL A E Elyse Lobach. Fluorination of Thiocarbonyl Compounds with Bis(2-methoxyethyl)aminosulfur Trifluoride (Deoxo-Fluor Reagent): A Facile Synthesis of gem-Difluorides[J]. Journal of Organic Chemistry, 2000, 65 16: 4783-5076. DOI:10.1021/jo000020j.
See also
Lastest Price from Bis(2-methoxyethyl)aminosulfur trifluoride manufacturers

US $2.20-8.80/kg2025-06-24
- CAS:
- 202289-38-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/kg2025-04-04
- CAS:
- 202289-38-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton