S-(+)-Methoprene: Discovery and Health Effects
General Description
S-(+)-Methoprene is a mimic or analog of the natural juvenile hormone (JH) of insects. The term juvenoid is often used to describe such compounds showing the same qualitative physiological activity against insect species as the natural JH. The commercialization of metho-prene in the mid-1970s was the first successful development of a biorational insecticide, a term coined by Zoecon Corporation scientists in 1974 to describe the approach of developing environmentally safe insecticides based on understanding the physiology of insects.1

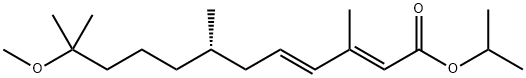
Figure 1. S-(+)-Methoprene
Discovery
Early Exploration of Juvenoid Analogues
The journey toward the discovery of S-(+)-Methoprene begins with the research on juvenile hormones (JHs), particularly the synthesis of terpenoid analogs before the actual structure of JH I was unveiled. Initial studies by chemists indicated that compounds like farnesol and farnesal exhibited weak juvenile hormone activity against larvae such as Tenebrio molitor, a finding that sparked further investigation. Despite the lack of potency in early analogs, such as farnesyl methyl ether, these efforts provided critical insights into the potential for creating more effective juvenoids. Researchers like Henrick and others contributed to this foundation by establishing connections between structural similarity and biological activity, including the work on natural products like (+)-juvabione, which showcased effective properties in certain insect species. 1
The Role of Zoecon Corporation and the Discovery
The publication of the structure of JH I led directly to the founding of Zoecon Corporation in August 1968. Zoecon was set up by Syntex Corporation to explore the concept that insect pests could be selectively controlled, without environmental concerns, by developing synthetic mimics of the natural JH. Since JH is involved in the regulation of physiological processes in insects, such as molting and metamorphosis, which do not occur in vertebrates, it was expected that selective insecticides could be developed. The natural JHs are neither stable enough under field conditions nor biologically active enough to be useful in commercial applications. The least stable functional groups in JH I in vivo were found to be the epoxide group and the methyl ester. Scientists at Zoecon set out to devise analogs that would be both more environmentally stable and more resistant to metabolism within the insect. Chemical modifications to the structure of JH I led to the discovery of the alkyl and alkynyl (2E,4E)-3,7,11-trimethyl-2,4-dodeca- dienoates. These juvenoids show very high activity against a wide spectrum of insect species.1
Commercial Success and Regulatory Milestones
At Zoecon Corporation, mosquito control was initially selected as a high priority target to demonstrate the practical feasibility of controlling insects with juvenoids. Much of the early structure-activity effort was concentrated against the yellow fever mosquito Aedes aegypti. Preparation of the first synthetic sample of racemic methoprene was completed in March 1971. After obtaining the initial bioassay results, it was immediately apparent that a significant discovery had been made. The LC 50 (concentration required to produce 50% inhibition of metamorphosis) against last larval instars of Ae. aegypti was 0.00017 ppm. Racemic methoprene was Zoecon Corporation’s first pro- prietary commercial compound and the first juvenoid to be registered for insect control. Initial sales began, on a limited experimental use basis, for floodwater mosquito control in August 1973.
In March 1975, racemic methoprene, as AltosidH SR-10 Insect Growth Regulator (IGR), sub-sequently designated Altosid Liquid Larvicide, received full commercial registration from the U.S. Environmental Protection Agency (USEPA) as a larvicide for the control of floodwater mosquitoes. This first registration of S-(+)-Methoprene was as a conventional chemical pesticide. Sub-sequently, the EPA reclassified methoprene as a biochemical pesticide. In addition to methoprene, two other analogs of this series, kinoprene and hydroprene were developed andregistered by Zoecon Corporation for insect control.1
Health Effects
Safety to Humans and Animals
S-(+)-Methoprene is of very low toxicity to humans and other vertebrates, and may be applied directly to pets, livestock and zoo animals for control of fleas, mites, and other parasites. There have been no reported cases of adverse effects to humans after accidental exposures to methoprene. It has been safely used in veterinary contexts for decades, including on pregnant and nursing mammals. Extensive review showed that the veterinary literature is devoid of studies showing developmental effects of methoprene used for parasite treatments on animals or their offspring. S-(+)-Methoprene and its breakdown products are sometimes used in studies attempting to disrupt the embryonic development of vertebrates, however levels necessary to produce developmental changes or other toxicities have been found to be over 100X that used in mosquito control or even greater. 2
Developmental Studies and Toxicity Levels
While S-(+)-Methoprene and its breakdown products are sometimes utilized in research aimed at disrupting embryonic development in vertebrates, the concentrations required to induce such effects are significantly higher—often over 100 times the amounts used in mosquito control. This indicates that S-(+)-Methoprene is unlikely to pose a risk to vertebrate development at the levels typically employed in pest management. Overall, the extensive safety data surrounding S-(+)-Methoprene supports its use as a reliable and low-risk insect growth regulator in both agricultural and veterinary contexts. 2
References:
[1] HENRICK C A. Methoprene.[J]. Journal of the American Mosquito Control Association, 2007, 23 2 Suppl. DOI:10.2987/8756-971X(2007)23[225:M]2.0.CO;2.[2] LAWLER S P. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control[J]. Ecotoxicology and Environmental Safety, 2017, 139: 1-496. DOI:10.1016/j.ecoenv.2016.12.038.
Lastest Price from S-(+)-METHOPRENE manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 65733-16-6
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $10.00/KG2025-04-21
- CAS:
- 65733-16-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt