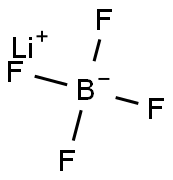Lithium Tetrafluoroborate: Applications in the Development of Lithium Batteries and its Toxicity
General Description
Lithium tetrafluoroborate (LiBF4) is widely used as an electrolyte for primary and secondary batteries. It can be used in rechargeable lithium-ion batteries. The electrolyte is a non-aqueous solution of LiBF4 in an organic medium. However, LiBF4 is toxic to humans and can cause severe skin burns, eye damage, and specific target organ toxicity, so it must be handled with care.

Figure 1. Lithium tetrafluoroborate
Applications in the Development of Lithium Batteries
There are two reasons for replacing volatile and flammable electrolytes in traditional lithium batteries: safety issues and increased specific energy. An ionic organic plastic crystal material (IOPC), n-ethyl-n-methylpyrrolidinium tetrafluoroborate [C2mpyr][BF4], was used as a solid electrolyte for lithium battery applications. The effect of 1 to 33 mol% lithium tetrafluoroborate (LiBF4) inclusion in [C2mpyr][BF4] was studied over a wide temperature range using differential scanning calorimetry (DSC), impedance spectroscopy, cyclic voltammetry, and full lithium|LiFePO4 cells. Results The order-of-magnitude increase in ionic conductivity observed at higher temperatures is most likely related to the increased mobility of Li ions in the highest plastic phase. At concentrations of 50 mol% LiBF4, the ionic conductivity of these solid composites is comparable to that of room temperature ionic liquids. Galvanostatic cycling of Li|Li symmetric cells demonstrates that the reversibility of the lithium metal redox reaction at this plastic crystal electrolyte interface is sufficient for lithium battery applications.
The use of alternative lithium salts in ester/fluoroethylene carbonate electrolytes: lithium tetrafluoroborate (LiBF4), lithium difluoro(oxalato)borate, and lithium hexafluorophosphate (LiPF6) showed improved low-temperature performance below 20 °C compared to LiPF6 in carbonate-based electrolytes. The ester-based formulations showed improved low-temperature performance at 20 °C compared to LiPF6 in carbonate-based electrolytes, but the room-temperature performance of the ester-based formulations showed significant capacity fade due to the instability of the solid electrolyte interphase (SEI). The researchers added electrolyte additives lithium bis(oxalate)borate, lithium difluorophosphate, and lithium bis(trimethylsilyl)phosphate (LiTMSP) to graphite//LiNi0.6Co0.2Mn0.2O2 cells to improve the room temperature cycling stability of lithium tetrafluoroborate (LiBF4) ester-based electrolytes (methyl acetate/fluoroethylene carbonate-MA/FEC) without reducing the low temperature rate performance of the cells. The LiBF4-based ester electrolyte containing 1% LiTMSP has the highest reversible capacity at low temperatures (?20 °C), and the room temperature performance is significantly improved compared to the base ester electrolyte (LiBF4-MA) and is almost equivalent to the standard carbonate-based electrolyte. Electrochemical impedance spectroscopy shows that the improved electrochemical performance of 1% LiTMSP at 20°C is due to the reduced charge transfer resistance; X-ray photoelectron spectroscopy shows that the improved room temperature performance is due to the formation of a stable phosphate-rich SEI on the graphite surface.
Solid electrolytes were prepared by adding LiBF4 to the plastic phase of [N13pyr]BF4. The [N13pyr]BF4-LiBF4 electrolytes containing 8-20 wt% LiBF4 are solid at temperatures below 80 °C and have high ionic conductivity of ~10-3-10-2 S cm-1 at 60 °C. Based on the results of DSC and conductivity studies, a phase diagram of the [N13pyr]BF4-LiBF4 binary system was drawn and the formation of a new compound 3[N13pyr]BF4-2LiBF4 was proposed. The existence of the new phase was supported by X-ray diffraction data. To test the suitability of these materials for lithium batteries, electrochemical measurements were performed on cells with lithium electrodes. The electrochemical window was determined to be above 5 V. No pretreatment effect was observed compared to previous data obtained for similar systems. However, the solid electrolyte [N13pyr]BF4-LiBF4 system has high ionic conductivity and can be used in solid-state lithium-ion batteries.
Toxicity
Lithium tetrafluoroborate (LiBF4) can cause severe skin burns and eye damage; inhalation can cause corrosive damage to the upper respiratory tract and lungs; ingestion and skin absorption are also harmful. In clinical practice, LiBF4 is also a skin toxicant (skin burns), and inhalation of metal fumes or toxic gases and vapors can cause lung inflammation.
References:
[1] SHEKIBI Y, RüTHER T, HUANG J, et al. Realisation of an all solid state lithium battery using solid high temperature plastic crystal electrolytes exhibiting liquid like conductivity[J]. Physical Chemistry Chemical Physics, 2012, 13: Page 4305 to 4660. DOI:10.1039/C2CP24077G.[2] CHAMITHRI JAYAWARDANA, BRETT L. LUCHT* Lithium Tetrafluoroborate-Based Ester Electrolyte Formulations to Improve the Operating Temperature Range in NCM 622 || Graphite Li-Ion Batteries[J]. ACS Applied Energy Materials, 2023, 6 10: 5102-5627. DOI:10.1021/acsaem.3c00261.
[3] A. ULIHIN N U D Novozhilov. Solid Electrolytes in the N-Propyl-N-methyl-pyrrolidinium Tetrafluoroborate—Lithium Tetrafluoroborate System[J]. Batteries, 2023. DOI:10.3390/batteries9030167.
You may like
Lastest Price from Lithium tetrafluoroborate manufacturers
US $0.00/KG2025-11-21
- CAS:
- 14283-07-9
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons

US $0.00/KG2025-08-22
- CAS:
- 14283-07-9
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 2 tons/year