Levulinic Acid: The Versatile Compound Revolutionizing Chemistry
Introduction
In recent years, amidst growing concerns over environmental sustainability and the need for renewable resources, levulinic acid has emerged as a beacon of hope in the chemical landscape. Its five-carbon structure belies a wealth of possibilities, captivating the imaginations of researchers and industry experts alike. As the world seeks greener alternatives and innovative solutions, the spotlight shines ever brighter on levulinic acid, heralding a new era of chemical innovation and sustainable development[1].

Figure 1 Characteristics of Levulinic acid
Synthesis Method
Levulinic acid synthesis primarily involves the acid hydrolysis of hexoses, such as glucose or fructose, under mild conditions. This process, typically catalyzed by mineral acids or solid acid catalysts, yields levulinic acid along with formic acid and other by-products. Alternatively, levulinic acid can be produced through the oxidation of levulinic acid esters or the dehydration of hydroxyacetone.
Additionally, innovative approaches to levulinic acid synthesis have been explored, including enzymatic catalysis and biomass conversion techniques. These methods offer sustainable pathways to produce levulinic acid, minimizing environmental impact while maximizing efficiency. By harnessing the power of biocatalysts or utilizing renewable feedstocks, researchers aim to enhance the scalability and eco-friendliness of levulinic acid production, paving the way for a more sustainable future in chemical synthesis.
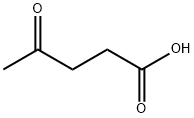
Composition
Furthermore, the molecular structure of levulinic acid endows it with remarkable versatility and reactivity, making it a cornerstone of modern chemistry. Its five-carbon backbone, adorned with a carboxylic acid group and a ketone moiety, forms the basis for its multifaceted applications. This distinctive arrangement not only imparts levulinic acid with reactivity but also enables facile modifications, driving innovation across industries. As scientists delve deeper into its molecular intricacies, new avenues for harnessing its potential continue to unfold, promising breakthroughs in fields ranging from pharmaceuticals to renewable energy.
Applications
Levulinic acid finds extensive applications across various industries, including:
Chemical Intermediates: Levulinic acid serves as a key building block for the synthesis of numerous chemicals, including pharmaceuticals, fragrances, and polymers.
Biofuel Production: Its conversion into levulinic acid esters or levulinate salts presents a promising avenue for biofuel production, offering a renewable and sustainable alternative to fossil fuels.
Food and Flavor Additives: Levulinic acid and its derivatives are utilized as food preservatives, flavor enhancers, and sweetening agents, contributing to the food industry's innovation.
Material Science: Levulinic acid-derived polymers exhibit desirable properties, making them suitable for applications in coatings, adhesives, and composite materials.
Toxicity
While levulinic acid is generally considered safe for most applications, prolonged or excessive exposure may pose health risks. Inhalation or ingestion of concentrated levulinic acid solutions can irritate the respiratory and digestive systems, leading to discomfort or adverse effects. As with handling any chemical substance, appropriate safety precautions must be observed to minimize potential hazards.
Moreover, it's essential to recognize that while levulinic acid is widely regarded as safe for many uses, prudent handling practices are imperative to mitigate potential risks. Concentrated solutions of levulinic acid, if inhaled or ingested, have the potential to irritate delicate respiratory and digestive tissues, potentially causing discomfort or adverse reactions. Therefore, stringent adherence to safety protocols, including the use of personal protective equipment and proper ventilation, is paramount when working with this compound. By exercising caution and implementing robust safety measures, the potential hazards associated with levulinic acid can be effectively managed, ensuring the well-being of workers and the environment alike.
Storage Considerations
Levulinic acid should be stored in a cool, dry place away from direct sunlight and sources of ignition. It is recommended to store levulinic acid in tightly sealed containers made of compatible materials, such as glass or polyethylene, to prevent contamination and degradation. Additionally, proper labeling and segregation from incompatible substances are essential to ensure safe handling and storage practices.
In conclusion, levulinic acid stands as a testament to the ingenuity of chemical innovation, offering a multitude of possibilities for advancing various industries while addressing sustainability concerns. As researchers continue to explore its potential and refine synthesis methods, levulinic acid is poised to play an increasingly pivotal role in shaping the future of chemistry and beyond[2].
References
[1]Bozell J J, Moens L, Elliott D C, et al. Production of levulinic acid and use as a platform chemical for derived products[J]. Resources, conservation and recycling, 2000, 28(3-4): 227-239.
[2]Leonard R H. Levulinic acid as a basic chemical raw material[J]. Industrial & Engineering Chemistry, 1956, 48(8): 1330-1341.
Related articles And Qustion
Lastest Price from Levulinic acid manufacturers

US $0.00/kg2025-04-21
- CAS:
- 123-76-2
- Min. Order:
- 25kg
- Purity:
- 98%min
- Supply Ability:
- 1000kg

US $100.00/kg2025-04-21
- CAS:
- 123-76-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200TON