Levulinic acid: synthesis and applications in drug synthesis
General Description
The synthesis pathway of Levulinic acid involves converting monosaccharides like glucose and fructose into the intermediate 5-hydroxymethylfurfural (HMF) through acid-catalyzed dehydration reactions. HMF is then hydrolyzed to prepare levulinic acid, with a maximum yield of around 81% mol when using glucose or fructose as starting materials. Levulinic acid finds applications in drug synthesis, including direct participation in drug synthesis, synthesis of related derivatives such as 5-aminolevulinic acid (ALA), and utilization as a chemical modifier and linker in targeted drug delivery systems. Levulinic acid also exhibits versatile reactivity, allowing for the formation of diverse functional groups such as indole and pyridazine, simplifying the synthesis of complex drug molecules. Additionally, levulinic acid can act as a protective group, enabling its involvement in subsequent reactions for the synthesis of various drugs.

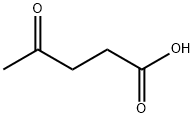
Figure 1. Levulinic acid
Synthesis
The synthesis pathway of levulinic acid involves the conversion of monosaccharides, such as glucose and fructose, into the intermediate HMF. This is achieved through acid-catalyzed dehydration reactions. Subsequently, HMF is hydrolyzed to prepare levulinic acid. The yield of evulinic acid from hexoses, specifically glucose and fructose, can reach up to 81% mol depending on the catalyst properties and reaction time. Polymerized carbohydrates can be enzymatically or acid catalyzed to convert into hexoses. These hexoses are then dehydrated to form HMF using an acid catalyst. The conversion of HMF to levulinic acid is considered the most suitable route for industrial-scale production. 1
![Article illustration]() Applications in drug synthesis
Applications in drug synthesis
Directly participate in synthesis
Levulinic acid can serve as a raw material for synthesizing drugs directly. For example, levulinic acid is used in the synthesis of a pH-responsive docetaxel-conjugated poly(lactic acid)-polyethyleneglycol (PLA-PEG) micellar formulation. The DTX conjugated polymeric micelles show targeted drug delivery capabilities through folate conjugation. They have a size of 181 nm and a critical micelle concentration of 5.18 μg/ml. DTX release from the micelles is pH-dependent, with significant differences between pH 5.0 and pH 7.4. The sustained drug release contributes to decreased cytotoxicity compared to free DTX. This pH-responsive nanoconjugate has potential for targeted anticancer drug delivery. 2
Synthesis of related derivatives
By synthesizing derivatives such as ALA, researchers have made significant strides in understanding and harnessing the therapeutic potential of this compound. ALA has been found to play a remarkable role in inducing endogenous porphyrin biosynthesis, particularly in Friend erythroleukemic cells. 3
As a chemical modifier and linker
Acylated hydroxyl groups are used to synthesize various amphiphilic HPMA copolymers for the targeted release of doxorubicin. Polymer conjugates bearing HA oligomers at the size of 10 oligosaccharides and above (HA(10-14)) bind actively and profoundly to CD44-overexpressing ovarian cancer cells (SK-OV-3) and internalize to the greatest extent relative to HA-polymer conjugates of 8 oligomers and below (HA(4-8)). The HA-modified HPMA-copolymer PTX conjugate (P-(HA)(34)-PTX) exhibited 50-times higher cytotoxicity towards CD44-overexpressing cells relative to the control, non-targeted, HPMA-copolymer PTX conjugate (P-PTX). P-(HA)(34)-PTX was significantly more toxic than the non-targeted P-PTX in cells expressing high levels of CD44. 4
Synthetic drug functional group
Levulinic acid possesses the remarkable ability to undergo acylation and esterification reactions, giving rise to diverse functional groups such as indole and pyridazine. This versatile reactivity not only simplifies the synthesis of complex drug molecules but also contributes to cost reduction in the pharmaceutical industry. One illustrative application of levulinic acid lies in the synthesis of pyridazine-functionalized derivatives for the treatment of triple-negative breast cancer. 5
As a protective group
Levulinic acid has the capability to selectively attach protective groups onto the hydroxyl or carboxyl positions of levulinic acid. This functionalization enables its involvement in subsequent reactions for the synthesis of various drugs. One notable application involves the utilization of levulinic acid as a protective agent for the 20-OH and 30-OH groups of vidarabine, leading to the synthesis of 50-O-D-valyl ester derivatives with potential antiviral activity against herpes viruses. 6
Reference
1. Mukherjee A, Dumont MJ, Raghavan V. Sustainable production of hydroxymethylfurfural and levulinic acid: challenges and opportunities. Biomass Bioenergy, 2015, 72:143-183.
2. Hami Z, Amini M, Ghazi-Khansari M, et al Synthesis and in vitro evaluation of a pH-sensitive PLA-PEG-folate based polymeric micelle for controlled delivery of docetaxel. Colloids Surf B, 2014, 116:309–317.
3. Malik Z, Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer, 1987, 56(5):589–595.
4. Journo-Gershfeld G, Kapp D, Shamay Y, Kope?ek J, David A. Hyaluronan oligomers-HPMA copolymer conjugates for targeting paclitaxel to CD44-overexpressing ovarian carcinoma. Pharm Res, 2012, 29(4):1121-1133.
5. Tao R, Gao M, Liu F, et al Alleviating the liver toxicity of chemotherapy via pH-responsive hepatoprotective prodrug micelles. ACS Appl Mater Interfaces, 2018, 10(26):21836–21846.
6. Dogan IS, Sarac S, Sari S, et al New azole derivatives showing antimicrobial effects and their mechanism of antifungal activity by molecular modeling studies. Eur J Med Chem, 2017, 130:124–138.
Related articles And Qustion
Lastest Price from Levulinic acid manufacturers

US $0.00/kg2025-04-21
- CAS:
- 123-76-2
- Min. Order:
- 25kg
- Purity:
- 98%min
- Supply Ability:
- 1000kg

US $100.00/kg2025-04-21
- CAS:
- 123-76-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200TON