Potassium Sorbate: A Comprehensive Insight into its Synthesis, Composition, Applications, Toxicity, and Safety Precautions
Potassium sorbate is obtained by the reaction of sorbic acid with potassium carbonate or potassium hydroxide. The structure of sorbic acid is similar to that of small molecule conjugated fatty acids.
The conjugated structure of fatty acids is -CH=CH-CH=CH-. This continuous carbon-carbon double bond structure (-C=C-) is very unstable and can be easily metabolized into carbon dioxide and water in the human body, which lays the foundation for the safety of potassium sorbate.
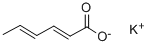
The chemical formula of potassium sorbate: CH3-CH=CH-CH=CH-COOK

Figure 1 Characteristics of Potassium sorbate
Characteristics of potassium sorbate
Sorbic acid and potassium sorbate have the same preservative properties, but the structure of sorbic acid is similar to that of fatty acids. Its solubility in water is average. After being made into potassium sorbate, it can be better dissolved in water.
Potassium sorbate is white or off-white granules or powder, odorless or slightly odorous, and stable to light and heat.
Potassium sorbate is easily oxidized because it contains two unsaturated conjugated double bonds, especially the double bonds far away from the carboxyl group (-COOH). The color of oxidized potassium sorbate will become darker.
Safety of potassium sorbate
From the structure of potassium sorbate, its main structure is a small molecule conjugated fatty acid structure, which is easily metabolized into carbon dioxide and water in the human body, so it is relatively safe.
The Joint Expert Committee on Food Additives (JECFA) jointly established by the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) has conducted multiple safety assessments on potassium sorbate, and the results show that potassium sorbate is safe to use at standard doses. The U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) also consider potassium sorbate to be a safe food additive.
According to current scientific research data, potassium sorbate is not listed as a Class I carcinogen. The International Agency for Research on Cancer (IARC) classifies potassium sorbate as Class 3, which means that the existing evidence is insufficient to consider it carcinogenic to humans.
You may like
Related articles And Qustion
Lastest Price from Potassium sorbate manufacturers

US $6.00/kg2025-04-21
- CAS:
- 24634-61-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $0.00/KG2025-04-21
- CAS:
- 24634-61-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt