Letermovir: Pharmacodynamics, Pharmacokinetics and Adverse Effects
General Description
Letermovir is a potent antiviral agent that selectively inhibits the cytomegalovirus DNA terminase complex, particularly the pUL56 subunit, disrupting viral DNA processing and packaging. It shows efficacy against resistant strains and has a high bioavailability of 94 percent. While letermovir generally exhibits favorable pharmacokinetics, its absorption and effectiveness can be influenced by concurrent use with ciclosporin, impacting dosing recommendations. Common adverse effects include gastrointestinal issues such as nausea and diarrhea, with overall tolerability comparable to placebo. Importantly, resistance mutations have been documented, but cross-resistance with other antiviral drugs is unlikely, affirming letermovir's utility in managing resistant cytomegalovirus infections.

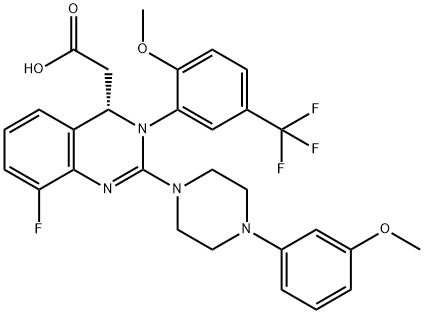
Figure 1. Letermovir
Pharmacodynamics
Mechanism of Action
Letermovir is a potent antiviral agent that specifically inhibits the cytomegalovirus (CMV) DNA terminase complex, particularly targeting the pUL56 subunit. This inhibition interferes with the processing and packaging of viral DNA, ultimately preventing the cleavage of viral DNA concatemers and the formation of mature virions. Unlike conventional antiviral drugs such as ganciclovir and ci-dofovir that target viral DNA polymerase, letermovir utilizes a unique mechanism. This distinction is critical in understanding why letermovir remains effective in cases where drug resistance has developed against traditional CMV treatments. In cell culture models, letermovir has demonstrated a median effective concentration of 2.1 nmol per liter against various clinical CMV isolates, showcasing its potency against this challenging virus. 1
Resistance Mechanisms and Clinical Implications
Resistance to letermovir has been observed, particularly involving mutations within the pUL56 subunit. Specific substitutions in the amino acid sequence of pUL56 have been identified in clinical studies, suggesting that these alterations can impact the susceptibility of CMV to letermovir. Notably, variations such as pUL56 V236M and C325W were documented in patients who experienced treatment failure during clinical trials. However, it is essential to note that cross-resistance between letermovir and other antiviral drugs targeting different viral mechanisms is unlikely. For instance, while letermovir is effective against strains resistant to CMV DNA polymerase inhibitors, the reverse is also true. In practical applications, such as in a lung transplant recipient with multi-drug resistant CMV, letermovir has successfully achieved rapid and lasting resolution of the infection, indicating its significant role in treating resistant strains of CMV. 1
Pharmacokinetics
Pharmacokinetics in Healthy Volunteers
Letermovir exhibits favorable pharmacokinetic properties, characterized by rapid absorption with a median time to peak plasma concentration ranging from 45 minutes to 2.25 hours. The absolute bioavailability of letermovir is notably high, reaching 94 percent across an oral dose range of 240 to 480 milligrams when taken without ciclosporin. The pharmacokinetics of letermovir demonstrate greater than dose-proportional behavior for both single and multiple administered doses, either orally or intravenously. Furthermore, letermovir demonstrates significant protein binding at about 99 percent across various plasma concentrations, indicating strong interactions with plasma proteins. It takes approximately nine to ten days to reach steady-state concentrations, which is crucial for maintaining effective therapeutic levels. 2
Impact of Co-Administration on Letermovir Pharmacokinetics
Letermovir is an inhibitor of cytomegalovirus terminase. This inhibition interferes with the maturation of viral DNA.
The bioavailability of the letermovir tablets in patients who have had a stem cell transplant is influenced by ciclosporin. Lower doses are used in patients taking ciclosporin as the bioavailability is 85% compared with 35% in those taking letermovir alone. This is because ciclosporin is an inhibitor of organic ion transporters. Although little of the letermovir molecule is metabolised, it can inhibit cytochrome P450 3A. This creates the potential for interactions with drugs such as midazolam. Although not all drugs have been studied, other commonly used medicines that may interact with letermovir include statins, proton pump inhibitors, phenytoin and warfarin. Co-administration with ergot alkaloids, pimozide, and ciclosporin with simvastatin is contraindicated.
Most of the dose of letermovir is excreted in the faeces. It should not be used in severe hepatic impairment, or moderate impairment if the patient also has moderate or severe renal impairment.2
Adverse Effects
Letermovir has generally been well tolerated in clinical trials, with gastrointestinal adverse events being the most prevalent. Common adverse events associated with letermovir include nausea, which occurred in 27 percent of recipients compared to 23 percent of those receiving a placebo, and diarrhea, reported in 26 percent versus 24 percent, respectively. Other notable adverse events include vomiting (19 percent), cough (14 percent), peripheral edema (14 percent), headache (14 percent), fatigue (13 percent), and abdominal pain (12 percent). Cardiac adverse events, primarily tachycardia and atrial fibrillation, were observed but were primarily mild to moderate in severity. Importantly, no cases of myelotoxicity or nephrotoxicity were reported. Although 13 percent of letermovir recipients discontinued treatment due to adverse events, this rate is comparable to the 12 percent observed in the placebo group. Furthermore, adverse events are unlikely to be mechanism-based, given that letermovir specifically targets the cytomegalovirus DNA terminase complex, which lacks parallels in mammalian physiology. 1
References:
[1] KIM E S. Letermovir: First Global Approval.[J]. Drugs, 2018, 78 1. DOI:10.1007/s40265-017-0860-8.[2] Letermovir for cytomegalovirus prophylaxis[J]. Australian Prescriber, 2019, 42 1: 107-107. DOI:10.18773/austprescr.2019.032.
Lastest Price from LeterMovir manufacturers
US $0.00/kg2025-11-27
- CAS:
- 917389-32-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/kg2025-04-21
- CAS:
- 917389-32-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 580kg