Lenvatinib: mechanism of action and anti-cancer therapy
General Description
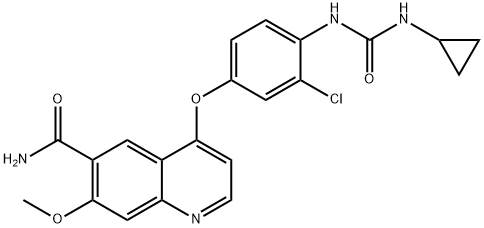
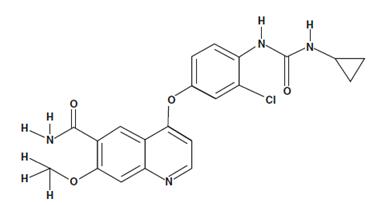
Lenvatinib is a potent inhibitor of the VEGF/VEGFR signaling pathway, which plays a crucial role in tumor angiogenesis. It blocks VEGFR tyrosine kinase activity, inhibiting endothelial and cancer cell proliferation, angiogenesis, and tumor growth. Lenvatinib also has the potential to affect the FGF/FGFR and RET signaling pathways, enhancing its antitumor activity in various cancers. Lenvatinib is used as an anti-cancer therapy for hepatocellular carcinoma, thyroid carcinoma, and thymic carcinoma.
Figure 1. Capsules of lenvatinib
![Article illustration]()
![Article illustration]() Mechanism of action
Mechanism of action
![Article illustration]() Inhibition of the VEGF/VEGFR signaling pathway
Inhibition of the VEGF/VEGFR signaling pathway
Lenvatinib is a potent inhibitor of the VEGF/VEGFR signaling pathway, which plays a crucial role in tumor angiogenesis. The overexpression of VEGF by tumor cells leads to the activation of VEGFR-2 on vascular endothelial cells and HCC cells, thereby inducing endothelial cell proliferation, vascular permeability, and HCC cell proliferation via downstream signaling pathways. Lenvatinib inhibits this pathway by blocking VEGFR tyrosine kinase activity, which prevents ligand binding and activation of signal transduction pathways such as Ras/MAPK, PI3K/AKT and PLCγ, resulting in the inhibition of endothelial and HCC cell proliferation, angiogenesis, and tumor growth. 1
Potential effect on the FGF/FGFR signaling pathway
Lenvatinib has the potential to affect the FGF/FGFR signaling pathway. Abnormally activated FGF signaling promotes tumor angiogenesis and progression by activating downstream pathways such as Ras/MAPK and PI3K/AKT. In HCC, aberrant expression of FGF19/FGFR4 contributes to tumor progression by activating downstream signaling pathways. Lenvatinib has been found to significantly inhibit the proliferation of FGF19- and FGFR-overexpressing cell lines in vitro and in vivo, potentially through its ability to block FGF binding to FGFR and subsequent activation of downstream pathways. Additionally, lenvatinib's dual inhibition of both the VEGFR and FGFR pathways enhances its antitumor activity in HCC by inhibiting angiogenesis and promoting cell death-related cleavage while also maintaining cell survival under nutrient-deficient conditions. 2
Inhibition of the RET signaling pathway
Lenvatinib has the potential to inhibit the RET signaling pathway, which can promote tumor cell proliferation through mutation or chromosomal rearrangement. RET activation occurs after ligand binding and receptor dimerization, leading to trans-autophosphorylation of specific tyrosine residues and recruitment of adaptor proteins that connect RET to intracellular pathways, including RAS/MAPK and PI3K/AKT, which promote cell growth, proliferation, survival, and differentiation. Lenvatinib can directly inhibit cell proliferation by blocking RET phosphorylation in vitro and in vivo, exhibiting antitumor activity in tumor models with RET gene fusion via inhibition of signals downstream of RET. Therefore, lenvatinib has the potential to be an effective treatment option for tumors with dysregulated RET signaling. 3
Anti-cancer therapy
Hepatocellular carcinoma
Lenvatinib metabolism is altered by hepatic function in patients with liver cancer, requiring a specific dosage regimen. A study found optimal starting doses of lenvatinib for advanced hepatocellular carcinoma based on liver function and body weight. Patients with Child-Pugh A liver function should take 12 mg daily while those with Child-Pugh B should take 8 mg daily. Pharmacokinetic analyses showed that dosing based on body weight could be effective. For those under 60 kg, the recommended starting dose is 8mg daily, while those over 60 kg should start at 12 mg daily. The dosing regimen in this study was applied in other study. 4
Thyroid carcinoma
Simulations predicted tumor response for different lenvatinib dosage regimens in patients with radioiodine-refractory differentiated thyroid carcinoma. A study compared the efficacy and safety of the recommended starting dose of 24 mg/day versus an 18 mg/day starting dose in a post-approval study. The 18 mg/day dose failed to demonstrate noninferiority based on ORR at week 24, supporting the use of the 24 mg/day starting dose for this indication. Safety profiles were comparable between the two arms and consistent with the known safety profile of lenvatinib in this patient population. 5
Thymic carcinoma
In a single-arm, multicenter, phase II trial known as the REMORA study, the efficacy and safety of lenvatinib were investigated in 42 Japanese patients with unresectable advanced or metastatic thymic carcinoma who had previously undergone platinum-based chemotherapy. Lenvatinib was administered at a starting dose of 24 mg/day. The objective response rate (ORR) was found to be 38%, with 16 out of 42 patients exhibiting a response to therapy (90% CI, 25.6–52.0). The median progression-free survival (PFS) was 9.3 months, while the median overall survival (OS) had not yet been reached at the time of data cutoff. The most common grade 3 treatment-related adverse events were hypertension (64%) and palmar-plantar erythrodysesthesia syndrome (7%). These positive results from the REMORA study led to the approval of lenvatinib in Japan for the treatment of patients with unresectable thymic carcinoma. 6
Reference
1. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer, 2008, 122(3):664-671.
2. Sandhu DS, Baichoo E, Roberts LR. Fibroblast growth factor signaling in liver carcinogenesis. Hepatology, 2014, 59(3):1166-1173.
3. Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett, 2013, 340(1):97-103.
4. Kudo M, Finn RS, Qin S, et al Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet, 2018, 391:1163–1173.
5. Hayato S, Shumaker R, Ferry J, et al Exposure-response analysis and simulation of lenvatinib safety and efficacy in patients with radioiodine-refractory differentiated thyroid cancer. Cancer Chemother Pharmacol, 2018, 82:971–978.
6. Sato J, Satouchi M, Itoh S, et al Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 2020, 21:843–850.
You may like
Related articles And Qustion
See also
Lastest Price from Lenvatinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 417716-92-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/kg2025-05-17
- CAS:
- 417716-92-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg