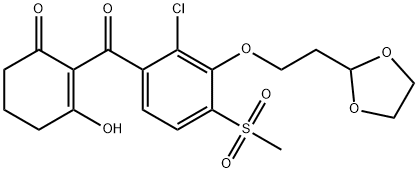
Lancotrione: mode of action, uses, and synthesis method
Description
Lancotrione was announced by Ishihara Sangyo Kaisha as a new herbicide in 2016. It was released in 2019 and commercialized in the form of sodium salts. It is closely related by structure to the established rice herbicide tefuryltrione. Both trione derivatives share the same herbicidal mode of action based on the inhibition of 4-hydroxyphenylpyruvate dioxygenase (HPPD). This enzyme catalyzes the formation of the plastoquinone precursor homogentisic acid. It is safe for paddy at the effective dose and has a good control effect on Echinochloacrusgalli, Scirpus, and Arrowhead.
Synthesis method
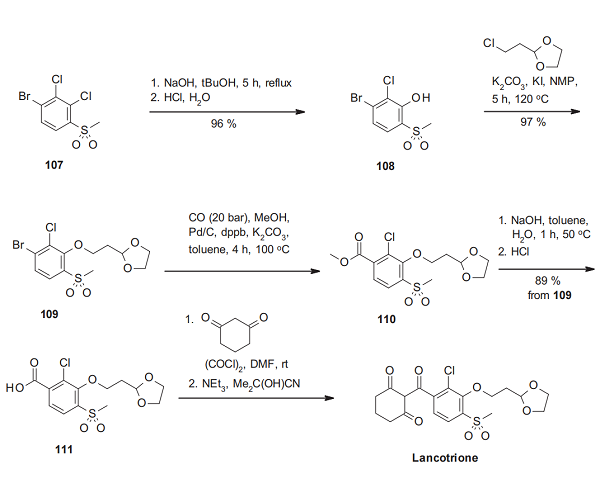
The synthesis of lancotrione starts from 1-bromo-2,3-dichloro- 4-methylsulfonylbenzene (107), which is refluxed with sodium hydroxide in tert-butanol to give 108[1]. Alkylation of this phenol derivative with 2-(2-chloroethyl)-1,3-dioxolane under basic conditions affords 109, converted in palladium-catalyzed carbonylation to the methyl ester 110. After ester saponification, the resulting benzoic acid derivative 111 delivers via O-acylation of 1,3-cyclohexanedione and, following cyanide-catalyzed rearrangement, the triketone lancotrione.
References
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.