A brief introduction to Bixlozone
Description
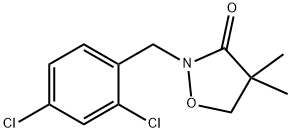
Bixlozone, 2-(2,4-dichlorobenzyl)-4,4-dimethylisoxazolidin-3-one, is a newly developed oxazole herbicide in the product of Overwatch by FMC Corporation, which is a newly registered active ingredient in April, 2020, and will be available for Australia in 2021.
Bixlozone was announced by FMC as a new herbicide in 2018. It was first reported in 1981 in the same patent application, containing the closely related and established selective soybean herbicide clomazone. Revisiting some data at a later stage showed that whilst bixlozone exhibits effective control of mono- and dicotyledonous weeds, its selectivity on grassy monocot crops, such as corn, rice, sorghum, barley, rye, and oilseed rape was also attractive. Moreover, the bixlozone exhibits reduced volatility compared to the closely related clomazone, which results in less unfavourable drift effects. Both compounds suppress the biosynthesis of chlorophyll and other plant pigments by inhibiting 1-deoxy-D-xylulose-5-phosphate synthase.

Toxicity
Bixlozone is mainly used to inhibit broadleaf weed and grassy weed growth by inhibiting 1-deoxy-d-xylulose 5-phosphate synthase, resulting in the disruption of plastid isoprenoid biosynthesis. According to the public release summary on evaluating the new active bixlozone from the Australian Pesticides and Veterinary Medicines Authority, the acute toxicity results showed that bixlozone had low acute oral, dermal, and inhalational toxicity[1]. The dietary risk assessment results showed that chronic dietary exposure to bixlozone is acceptable. However, on application to different matrices, the mainly corresponding metabolites form, including 2,4-dichlorobenzoic acid, 3-hydroxy-propanamide-bixlozone (also known as N-(2,4-dichlorobenzyl)−3-hydroxy-2,2-dimethylpropanamide), and 5′-hydroxy- bixlozone (also known as 2-(2,4-dichloro-5-hydroxybenzyl)-4,4-dimethylisoxazolidin-3-one).
Synthesis method
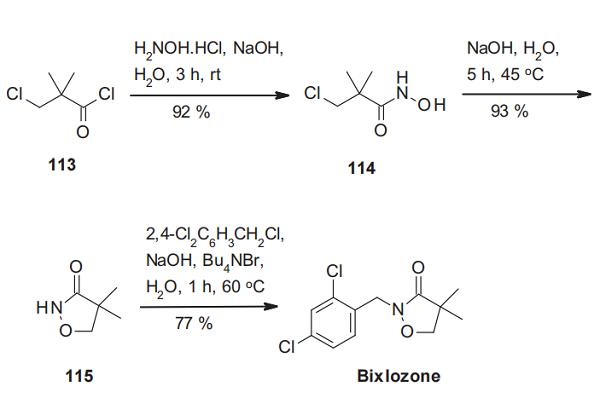
The synthesis of bixlozone starts with the transformation of 3- chloro-2,2-dimethylpropionyl chloride (113) with hydroxylamine hydrochloride to the hydroxamic acid 114, which is then cyclized under strongly primary conditions to the isoxazolidinone 115. Alkylation of 115 with 2,4-dichlorobenzyl chloride under phase-transfer conditions affords bixlozone[2].
References
[1] Congdi Li. “Simultaneous determination of the herbicide bixlozone and its metabolites in plant and animal samples by liquid chromatography–tandem mass spectrometry.” Journal of separation science 44 4 (2020): 822–832.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.