L-Serine: Biosynthesis and Potential Therapeutic Role in Diabetes
General Description
The biosynthesis of L-Serine is regulated by three main enzymes, and its production is responsive to cellular demands, with upregulation observed in rapidly proliferating tissues and in cell cultures of L-Serine starved cells. L-Serine has been associated with insulin sensitivity, with studies showing that reduced expression of PSAT and lower levels of liver L-Serine are linked to insulin resistance. Additionally, L-Serine has been implicated in various metabolic pathways and has potential therapeutic implications for diabetes and its related complications. Research suggests a potential role for L-Serine in the treatment and management of diabetic retinopathy, as well as in promoting neuronal growth and differentiation. Furthermore, L-Serine's involvement in the synthesis of phosphatidylserine and sphingolipids could be crucial in diabetes development. Altered concentrations of L-Serine have been observed in patients with both type 1 and type 2 diabetes, and its potential role in modulating the production of deoxysphingolipids offers promising avenues for therapeutic intervention in diabetic neuropathy. Overall, the multifaceted roles of L-Serine in cellular metabolism and neurological function make it a compelling candidate for further exploration as a therapeutic target in the management of diabetes and its associated complications.

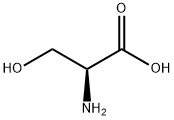
Figure 1. L-Serine
Biosynthesis
The biosynthesis of L-Serine involves a series of enzymatic reactions catalyzed by three main enzymes: phosphoglycerate dehydrogenase (PGDH), phosphoserine aminotransferase (PSAT), and phosphoserine phosphatase (PSP). Among these, PSP is considered the rate‐limiting step in serine biosynthesis, thereby regulating the overall flux. The regulation of L-Serine synthesis is responsive to cellular demands, with upregulation of the enzymes observed in rapidly proliferating tissues and in cell cultures of L-Serine starved cells. Interestingly, studies have shown that a protein‐restricted diet in rats can lead to a doubling of L-Serine concentration in the blood, attributed to increased de novo synthesis. Furthermore, two enzymes involved in L-Serine biosynthesis have been associated with insulin sensitivity. Reduced expression of PSAT and lower levels of liver L-Serine have been observed in leptin receptor‐deficient (db/db) mice and high‐fat diet (HFD)‐induced diabetic mice. Subsequent research demonstrated that overexpression of PSAT led to improved insulin signaling and sensitivity. Conversely, inhibition of PSP was found to cause inappropriate serine dephosphorylation of substrates, resulting in insulin resistance in rat adipocytes. Overall, the biosynthesis of L-Serine is intricately regulated in response to cellular demands and has significant implications for insulin sensitivity. Understanding the molecular mechanisms underlying L-Serine biosynthesis and its impact on metabolic pathways may provide valuable insights into potential therapeutic targets for conditions related to insulin resistance and metabolic disorders. 1
Potential Therapeutic Role in Diabetes
L-Serine, a non-essential amino acid, has been implicated in various metabolic pathways, serving as a constituent of proteins and playing a crucial role in neuronal function, growth, and differentiation. Recent studies have suggested a potential therapeutic role for L-Serine in the treatment and management of diabetes and its related complications. One significant aspect to consider is the relationship between L-Serine and the development of diabetic retinopathy. Research has shown that D‐serine, an enantiomer of L-Serine, produced by serine racemase, is linked to reduced insulin secretion in mice and is associated with the development of diabetic retinopathy. Additionally, L-Serine acts as a neuronal trophic factor, promoting the growth, differentiation, and elongation of cultured neurons, which could have implications for neurological complications of diabetes. The involvement of L-Serine in the synthesis of phosphatidylserine and sphingolipids is particularly relevant to diabetes. Sphingolipids, in particular, are suggested to play a significant role in diabetes development, and L-Serine's role in their synthesis could be crucial. Furthermore, L-Serine also participates in the formation of NADPH, nucleotides, and serves as a carbon source for methylations, all of which have implications for cellular metabolism and proliferation in the context of diabetes and associated pathologies. Studies have observed altered concentrations of L-Serine in patients with both type 1 and type 2 diabetes, with reduced levels found in plasma. This reduction in L-Serine levels appears to be independent of insulin production, as evidenced by increased L-Serine levels in diabetic mice following streptozotocin treatment. The metabolic implications of these altered L-Serine levels in the context of diabetes warrant further investigation to understand their role in disease development and progression. A particularly intriguing aspect is the potential role of L-Serine in modulating the production of deoxysphingolipids, which are known to induce apoptosis in beta-cell lines and impair neuronal function. Patients with primary L-Serine deficiency have increased amounts of deoxysphingolipids, while L-Serine supplementation has been shown to reduce their concentration. Notably, deoxysphingolipids are enriched in plasma from type 2 diabetes patients, suggesting a potential link between altered L-Serine metabolism and diabetes-related complications. Furthermore, the association between SPT mutations, deoxysphingolipid accumulation, and neuropathy in diabetes highlights the potential therapeutic impact of modulating L-Serine levels. Studies in mouse models and diabetic rats have shown that increasing L-Serine concentration leads to a reduction in deoxysphingolipids and improved neuronal function, offering promising avenues for therapeutic intervention in diabetic neuropathy. In conclusion, the multifaceted roles of L-Serine in cellular metabolism, neuronal function, and sphingolipid synthesis make it a compelling candidate for further exploration as a therapeutic target in the management of diabetes and its associated complications. Further research into the precise mechanisms through which L-Serine influences diabetes pathogenesis and its potential for therapeutic intervention is warranted. 2
Reference
1. Fell DA, Snell K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem J. 1988; 256(1): 97-101.
2. Holm LJ, Buschard K. L-serine: a neglected amino acid with a potential therapeutic role in diabetes. APMIS. 2019; 127(10): 655-659.
You may like
Related articles And Qustion
Lastest Price from L-Serine manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 56-45-1
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 10 tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 56-45-1
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.5%
- Supply Ability:
- 10 TONS