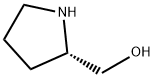
L-(+)-Prolinol: a chiral auxiliary
L-(+)-Prolinol is defined as a chiral auxiliary used in the preparation of cyclic P-chiral phosphonates in organic chemistry. It helps in fixing the configuration at the phosphorus atom and is essential for achieving high selectivity in catalytic reactions.

Preparation of L-(+)-Prolinol
In a 0.5 L autoclave, 60 g of L-pyroglutamic acid, 60 ml of acetic acid,120 ml of water was charged and 10 g of the molybdenum atom-modified ruthenium-carbon catalyst (ruthenium content: 5 wt%, molybdenum content: 2 wt%) prepared above was added and reacted for 20 hours under a hydrogen pressure of 7 MPa and a temperature of 100 ° C. Cool to room temperature, pressure relief, filtration. The filter cake ruthenium carbon catalyst sets for the next reaction, the filtrate was concentrated under reduced pressure, L-(+)-Prolinol obtained crude product about 60g. The crude product was distilled to give 44 g of L-(+)-Prolinol , 99.1% purity (GC), yield: 91%: 60 g L-pyroglutamic acid, 60 ml acetic acid and 120 ml water were added to a 0.5 L autoclave. In the meantime, 10g of molybdenum atom-modified ruthenium carbon catalyst recovered in the above experiment (recycled under the same reaction conditions for 5 times) was added. And the above reaction conditions are the same, Obtained crude and then after distillation L-(+)-Prolinol 45.8g, purity 99.4% (GC), yield: 95%.[1]
L-(+)-Prolinol as a highly enantioselective catalyst
Though many chiral amines such as ;l-proline and its derivatives have proven to be versatile catalysts in many reactions, ;L-(+)-Prolinol ;was seldom used as organocatalyst for reactions. Herein, we report the first ;L-(+)-Prolinol ;catalyzed asymmetric Michael addition of cyclohexanone to nitroolefins in the presence of benzoic acid to afford Michael adducts with high diastereoselectivities (87:13–>99:1) and enantioselectivities (82–96%).[2]
The activation of the donors and control of asymmetric induction most likely occur through the formation of enamine intermediates. ;Taking the proposed transition state of ;L-(+)-Prolinol -type catalyst ;into consideration, we envisioned that ;L-(+)-Prolinol -catalyzed reaction of cyclohexanone to nitrostyrene will most probably proceed through similar hydrogen bonding in the transition state. Furthermore, the development of the simple and commercially available ;L-(+)-Prolinol ;as a good organocatalyst will potentially reduce much hassle for stereoselective reactions. It would be an added advantage if the reaction could be carried out under solvent-free conditions. In view of the above advantages, we report the enantioselective organocatalytic Michael addition of cyclohexanone to nitroolefin using ;L-(+)-Prolinol ;as catalyst under neat conditions, as well as the scope of the above-mentioned reaction. The first ;L-(+)-Prolinol -catalyzed asymmetric Michael additions of cyclohexanone to nitroolefins in the presence of benzoic acid afforded the respective adducts in good to high yields with high diastereoselectivities and enantioselectivities. Regio-selectivity of this reaction was also demonstrated. Further investigations on the application of ;L-(+)-Prolinol in natural product synthesis is in progress.
L-(+)-Prolinol-Derived Surfactants as Antibacterial Nanocarriers
L-(+)-Prolinol-derived surfactants, which were shown to enhance the efficacy of DNA and other lipid drug-delivery systems, ;reduce the effective dose of some antibiotics ;and confer antibacterial activity to liposomal formulations. In a previous investigation, liposomes containing analogous structurally related ;L-(+)-Prolinol derivatives showed a good ability to efficiently deliver UA to ;S. aureus ;bacterial cells. The efficacy of the treatment of bacterial infection is seriously reduced because of antibiotic resistance; thus, therapeutic solutions against drug-resistant microbes are necessary. Nanoparticle-based solutions are particularly promising for meeting this challenge because they can offer intrinsic antimicrobial activity and sustained drug release at the target site. Herein, we present a newly developed nanovesicle system of the quatsome family, composed of ;L-(+)-Prolinol-derived surfactants and cholesterol, which has noticeable antibacterial activity even on Gram-negative strains, demonstrating great potential for the treatment of bacterial infections. Nanoscale quatsomes formulated with structurally related ;L-(+)-Prolinol-derived surfactants were prepared and characterized. As a whole, the supramolecular nanometric structures containing longer chains (C14 and C16) were very stable and showed a good ability to entrap UA, demonstrating high potential as a drug-delivery system
References
[1] PANJIN GELIN KAIMO TECH - CN106748948, 2017, A
[2]Chua, P. J., Tan, B., Zeng, X. F., & Zhong, G. F. (2009). L-Prolinol as a highly enantioselective catalyst for Michael addition of cyclohexanone to nitroolefins. Bioorganic & Medicinal Chemistry Letters, 19(14), 3915-3918.
[3]Battista S, Köber M, Bellio P, Celenza G, Galantini L, Vargas-Nadal G, Fagnani L, Veciana J, Ventosa N, Giansanti L. Quatsomes Formulated with l-Prolinol-Derived Surfactants as Antibacterial Nanocarriers of (+)-Usnic Acid with Antioxidant Activity. ACS Appl Nano Mater. 2022 May 27;5(5):6140-6148.
You may like
See also
Lastest Price from L-(+)-Prolinol manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 23356-96-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+

US $0.00-0.00/kg2025-04-04
- CAS:
- 23356-96-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton