L-Cystine: Metabolism and Biological Functions
General Description
L-Cystine, a non-essential amino acid, is crucial for protein synthesis due to its sulfur-containing thiol group. It plays a key role in forming disulfide bridges for protein folding and stabilization. Metabolically, L-Cystine is synthesized from methionine and contributes to various cellular functions, producing important molecules like taurine and sulfate. Biologically, it is essential for protein structure, detoxification through glutathione synthesis, metabolic pathways involving coenzyme A, and immune system regulation. Overall, L-Cystine is a versatile amino acid with significant implications for various physiological processes and human health.

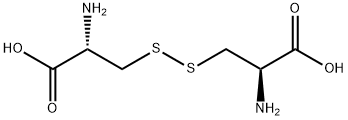
Figure 1. L-Cystine
Overview
L-Cystine is a vital non-essential amino acid crucial for protein synthesis. Its distinctive feature lies in the sulfur-containing thiol group (-SH) at the end of its side chain, endowing it with high reactivity. This reactivity underpins its diverse biological functions in humans. Notably, L-Cystine facilitates the formation of disulfide bridges, covalent bonds essential for the folding and stabilization of proteins' tertiary structures. This structural support is pivotal for the proper functioning of proteins in biological processes. The presence of conserved cysteine residues in protein motifs across all organisms suggests their early evolutionary utilization in enzyme catalysis, transcriptional regulation, protein folding, and the establishment of three-dimensional structures. This underscores the fundamental role of L-Cystine in the molecular machinery of life, making it a cornerstone in understanding and harnessing biological processes. 1
Metabolism
L-Cystine, an essential amino acid, undergoes a series of intricate metabolic processes within the body, crucial for various cellular functions. Initially synthesized from methionine, L-Cystine is formed through two distinct chemical reactions. The first step involves a transmethylation reaction, yielding homocysteine as a byproduct. Subsequently, homocysteine undergoes a transsulfuration reaction, resulting in the production of cysteine. Once synthesized, cysteine can be assimilated through various pathways, depending on the cellular requirements, leading to the formation of sulfur compounds. Among the metabolites derived from cysteine assimilation, sulfinate is a prominent molecule. Sulfinate can further metabolize to generate sulfinylpyruvate and pyruvate or hipotaurine and taurine. Taurine, a significant intracellular molecule, plays diverse roles in biological processes. Although its exact biological function remains unclear, taurine is believed to be essential for nervous system functioning, particularly in brain development. Studies have shown elevated levels of taurine in the fetal brain, indicating its importance in early neurodevelopment. Additionally, taurine is involved in various other functions such as detoxification of certain intermediates and regulation of intracellular calcium levels. Furthermore, during the metabolism of L-Cystine, sulfinylpyruvate can undergo oxidation to sulfate, which is subsequently utilized in the synthesis of 3′-phosphoadenosine-5′-phosphosulfate. Additionally, several other vital reactions occur during L-Cystine metabolism, facilitating the synthesis of thiocysteine and the transfer of sulfur between molecules. In summary, the metabolism of L-Cystine involves a complex interplay of biochemical reactions, leading to the formation of crucial molecules like taurine and sulfate, essential for various physiological processes within the body. 2
Biological Functions
L-Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues that are linked by a disulfide bond. L-Cystine plays a crucial role in various biological functions and is essential in human health. A key aspect of L-Cystine's biological function is its role in the structure and stability of proteins. The disulfide bonds in L-Cystine are critical for maintaining the three-dimensional structure of many enzymes and structural proteins, influencing their stability and function. L-Cystine is also vital in detoxification processes. It acts as a precursor to glutathione, a significant antioxidant in the human body. Glutathione helps to combat oxidative stress by neutralizing free radicals and reactive oxygen species, thereby protecting cells from damage. This makes L-Cystine indispensable in maintaining cellular health and function. Furthermore, L-Cystine contributes to the synthesis of other vital substances, such as coenzyme A, which is essential for metabolic processes involving carbohydrates, proteins, and fats. L-Cystine's role in these metabolic pathways highlights its importance in energy production and resource distribution within the body. L-Cystine is also involved in the regulation of immune function. It supports the proliferation of lymphocytes and the production of cytokines, which are critical for effective immune responses. Additionally, L-Cystine influences the activity of leukocytes, including their movement and ability to combat pathogens. Overall, L-Cystine is a multifunctional amino acid with vital roles in protein structure, detoxification, metabolism, and immune system regulation, underscoring its importance in biological systems and human health. 2
Reference
1. Devlin TM. Textbook of Biochemistry with Clinical Correlations. 7th ed. John Wiley & Sons Inc.; Hoboken, NJ, USA: 2010. Chapters 3 and 9.
2. Clemente Plaza N, Reig García-Galbis M, Martínez-Espinosa RM. Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules. 2018 Mar 3; 23(3): 575.
You may like
See also
Lastest Price from L-Cystine manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 56-89-3
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.0%
- Supply Ability:
- 10 TONS

US $1.00/PCS2025-04-21
- CAS:
- 56-89-3
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt