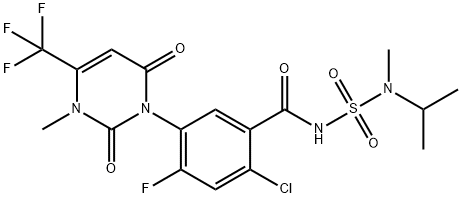
The toxicity of Saflufenacil
Introduction
Saflufenacil is a Group 14 protoporphyrinogen oxidase (PPO) inhibitor, also known as a cell membrane disruptor, herbicide primarily used to control grass and broadleaf weeds, as a pre-plant or pre-emergence application in corn, sorghum, soybean, small grains, and tree/nut/vine crops. It was first registered by the U.S. Environmental Protection Agency in 2009[1].

Mechanism of action
Saflufenacil controls many common annual broadleaf weeds, including acetolactate synthase-resistant biotypes, by inhibiting protoporphyrinogen oxidase (protox). Protox-inhibiting herbicides prevent the biosynthesis of chlorophyll and heme by competitive inhibition of protox[2]. As a result of protox inhibition, protoporphyrinogen IX (protogen) is not converted to protoporphyrin IX (proto) in chloroplasts and protogen accumulates until it leaks from chloroplasts into the cytoplasm. In the cytoplasm, protogen is converted to proto by enzymes in the cytoplasm. Proto reacts with light and oxygen to form radical singlet oxygen that causes lipid peroxidation.
Toxicity
Saflufenacil has low acute toxicity via the oral, dermal and inhalation routes of exposure (Toxicity category III or IV). It is slightly irritating to the eye (Toxicity category III). It is neither a dermal irritant nor a sensitizer.
The primary target organ is the hematopoietic system. PPO inhibition in mammals may disrupt heme synthesis, which in turn causes anemia. In the submitted studies, decreased hematological parameters [decreased red blood cells (RBC), decreased hematocrit (Ht), decreased mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular volume (MCV)] were observed at about the same dose level across species, except the dog, where effects were observed at a slightly higher dose. These effects occurred around the same dose level from short- to long-term exposures without increasing severity. Effects were also seen in the liver (increased weight, centrilobular fatty change, lymphoid infiltrate) in mice, the spleen (increased spleen weight and extramedullary hematopoiesis) in rats, and both these organs (increased iron storage in the liver and extramedullary hematopoiesis in the spleen) in dogs. These effects also occurred around the same dose level from short- to long-term exposures without increasing severity. No dermal toxicity was seen at the limit dose in a 28-day dermal toxicity study in rats.
Saflufenacil is classified as “Not Likely Carcinogenic to Humans” based on no evidence of increased incidence of tumours at the tested doses in rats and mice. Saflufenacil is weakly clastogenic in the in vitro chromosomal aberration assay in V79 cells in the presence of S9 activation; however, the response was not evident in the absence of S9 activation.
Rat metabolism data indicate that saflufenacil is well absorbed and rapidly excreted. The maximum concentration of saflufenacil in blood and plasma was reached within 1 hour (h) of dosing and declined rapidly after 24 h. Excretion of orally dosed saflufenacil was essentially complete within 96 h, with the majority eliminated within the first 24 to 48 h. There was a sex-dependent difference in the excretion of orally administered saflufenacil. The main route of elimination in male rats was via the feces, while urinary excretion was the major route of elimination in females. The sex-dependent excretion was more pronounced at the low-dose level than at the high-dose level. Also, males had significantly higher biliary excretion of saflufenacil residues than females. Exhalation was not a relevant excretion pathway of saflufenacil. At 168 h after dosing, saflufenacil residues remaining in tissues were very low and occurred mainly in carcass, liver, skin, and gut contents. The parent molecule and 3 major metabolites (M800H01, M800H03, and M800H07) were identified and isolated from urine and feces. Minor metabolites that were identified include M800H05, M800H16, M800H17, M800H18, M800M19, and M800M20. There were no significant gender differences in metabolic profiles. Saflufenacil was metabolized by three major transformation steps, which were demethylation of the uracil ring system, degradation of the N-methyl-N-isopropyl group to NH2, and cleavage of the uracil ring, forming a sulfonylamide group.
References
[1] Jared M. Roskamp, William G. Johnson, Gurinderbir S. Chahal. “Influence of Water Hardness and Co-applied Herbicides on Saflufenacil Efficacy.” Crop Management 11 1 (2012): 1–8.
[2] John C. Frihauf, Kassim Al-Khatib, Phillip W. Stahlman . “Saflufenacil absorption and translocation in winter wheat (Triticum aestivum L.).” Pesticide Biochemistry and Physiology 98 2 (2010): Pages 243-247.
[3] US EPA - Pesticides - Fact Sheet for Saflufenacil. https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-118203_01-Aug-09.pdf