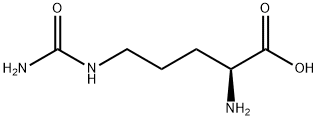
L-Citrulline:a naturally occurring amino acid
Introduction
L-Citrulline is a naturally occurring amino acid that plays a significant role in the urea cycle, a metabolic pathway in the body that helps remove ammonia, a waste product of protein metabolism. It is considered a non-essential amino acid, which means that the body can synthesize it on its own. L-Citrulline is primarily found in watermelon, but it is also available in supplement form. Current evidence suggests a BP-lowering effect of Cit and Arg, but more research is needed to solve the “l-arginine paradox” and understand the exact mechanism by which this occurs. The BP-lowering effect found in the above-presented meta-analyses for both Arg and Cit is based on several small trials with heterogenic study populations regarding baseline BP and comorbidity , in which highly variable intervention durations and dosages were used.
Application
L-Citrulline is a non-essential amino acid that plays a pivotal role in the urea cycle, a metabolic pathway responsible for removing toxic ammonia from the body. Its conversion into L-arginine can lead to increased nitric oxide production, promoting vasodilation and improved blood flow. This vasodilatory effect is one of the key mechanisms through which L-Citrulline is believed to influence exercise performance. Physical performance and exercise endurance are critical components of maintaining a healthy lifestyle. Athletes and fitness enthusiasts often seek ways to enhance their performance and reduce post-exercise fatigue and soreness. L-Citrulline, an amino acid found in watermelon and available as a dietary supplement, has emerged as a potential aid in achieving these goals. This paper examines the scientific evidence supporting the use of L-Citrulline in exercise-related contexts.
With global warming becoming of increasing concern, poultry farms are experiencing a concomitant increase in heat stress. Chickens are very sensitive to high ambient temperature (HT), so the development of novel nutrients that will help deal with the challenge posed by heat stress is vital. We revealed that L-citrulline can reduce body temperature in chickens. Orally administered L-citrulline solution has been found to provide heat tolerance in chickens and to result in reduced food intake. Heat exposure and oral administration of L-citrulline led to increased levels of plasma insulin, whereas heat stress led to a decline in plasma thyroxine. Dietary administration of L-citrulline was also shown to be effective to reduce heat stress in broiler chickens. Moreover, L-citrulline was found to be metabolized in the liver within 1 h of its administration, and in L-citrulline-treated broiler chicks, the Cit-Arginine cycle and the Krebs cycle were found to be active. L-citrulline has not yet been approved for inclusion in the poultry diet, so it is important to find alternative sources of L-citrulline. Taken together, these findings suggest that L-citrulline may serve as an important novel nutrient with the ability to produce heat tolerance in chickens under HT[1].
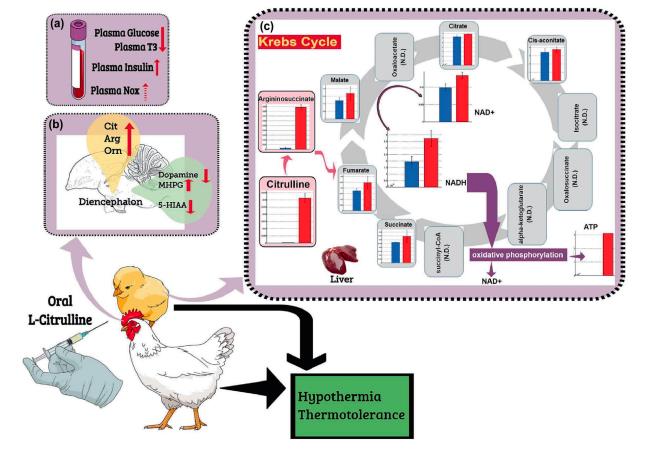
Figure 1 The role of plasma components (metabolites, hormones, and NO), brain monoamines, and liver metabolism in hypothermia and thermotolerance induced by oral L-citrulline. This schematic drawing shows the changes in different plasma hormones and metabolites in chicks and broilers following oral L-Cit and the consequences for hypothermia and thermotolerance
Foods rich in polyphenols and nitrate provide trivial benefits for endurance exercise performance, although these effects may be food dependent. Highly trained endurance athletes do not appear to benefit from consuming nitrate-rich foods but may benefit from polyphenol consumption. Further research into food sources, dosage and supplementation duration to optimise the ergogenic esponse to polyphenol consumption is warranted. Further studies should evaluate whether differential sex-based responses to nitrate and polyphenol consumption are attributable to physiological differences or sample size limitations[2].
Synthesis
L-Citrulline can be synthesized through several chemical reactions. One common method for the synthesis of L-Citrulline involves the use of starting materials like L-ornithine or L-arginine. Here's a simplified overview of the synthetic process:
Step 1: Protection of the Amino Groups The amino groups in the starting material (e.g., L-ornithine or L-arginine) are protected with a suitable protecting group, such as the Boc (tert-butyloxycarbonyl) group or the Fmoc (9-fluorenylmethyloxycarbonyl) group. This step prevents unwanted reactions from occurring at these sites during subsequent reactions. Step 2: Formation of Urea The protected amino group is reacted with an isocyanate, such as phosgene or another suitable reagent, to form the urea group. This step connects two of the three amino acid moieties present in L-Citrulline. Step 3: Removal of Protecting Groups The protecting groups are removed from the amino groups to expose the free amino groups. This step is typically carried out using suitable reagents, depending on the type of protecting group used. Step 4: Addition of the Third Amino Acid Moiety The remaining protected amino group is reacted with another amino acid, typically L-arginine, to form the third amino acid moiety in the L-Citrulline molecule. Step 5: Removal of All Protecting Groups All protecting groups are removed to obtain the final L-Citrulline molecule with free amino groups.
The synthesis of L-Citrulline is a complex organic chemistry process that involves multiple chemical reactions and steps. It requires careful control of reaction conditions and the use of protective groups to ensure that the desired product is obtained. The specific reagents and conditions used can vary depending on the synthetic route chosen by chemists. It's important to note that the industrial production of L-Citrulline is typically more efficient and cost-effective when performed at a larger scale, and it may involve variations in the synthetic process compared to laboratory-scale synthesis. The details of the process and the choice of reagents may be proprietary information held by manufacturers.
Safety
Nitric oxide (NO) is a well-known vasodilator produced by the vascular endothelium via the enzyme endothelial nitric oxide synthase (eNOS). The inadequate production of NO has been linked to elevated blood pressure (BP) in both human and animal studies, and might be due to substrate inaccessibility . This review aimed to investigate whether oral administration of the amino acids l-arginine (Arg) and l-citrulline (Cit), which are potential substrates for eNOS, could effectively reduce BP by increasing NO production. Both Arg and Cit are effective at increasing plasma Arg. Cit is approximately twice as potent, which is most likely due to a lower first-pass metabolism. While L-Citrulline is generally considered safe when taken within recommended dosages, it is essential to consult with a healthcare provider before starting any supplementation regimen. Some individuals may experience minor side effects or interactions with certain medications.
Reference
1. Chowdhury V S. L-Citrulline: A novel hypothermic amino acid promoting thermotolerance in heat-exposed chickens[J]. Anim Sci J, 2023,94(1):e13826.
2. Khalaf D, Kruger M, Wehland M, et al. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure[J]. Nutrients, 2019,11(7).
You may like
Related articles And Qustion
See also
Lastest Price from L-Citrulline manufacturers

US $1200.00-1100.00/ton2025-10-31
- CAS:
- 372-75-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $15.00/KG2025-06-06
- CAS:
- 372-75-8
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 1 ton