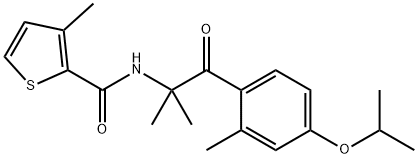
Isofetamid: Synthesis and Introduction
Synthesis of Isofetamid
Isofetamid is synthesised using m-cresol as a raw material by chemical reaction. The specific synthesis steps are as follows:
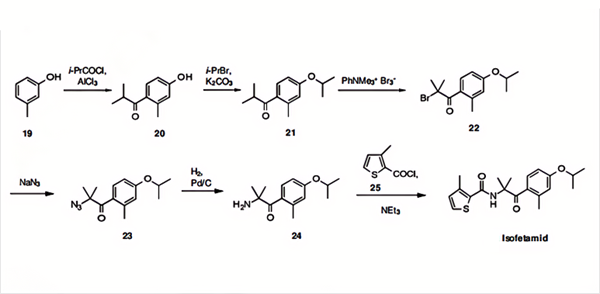
The phenethylamine moiety of isofetamid is prepared in five steps starting from m-cresol (19) by Friedel-Crafts acylation to 20, etherification of the phenol function to 21, α-ketobromination to 22, bromo-azido exchange to 23, and azide reduction to the isofetamid amine 24. This important intermediate is then converted to isofetamid (III) by amidation with 3-methylthiophene-2-carbonylchloride (25).
Introduction of Isofetamid
Isofetamid was announced by Ishihara Sangyo Kaisha (ISK) in 2012. After fluopyram 18, isofetamid is the second compound of this class bearing a phenethylamide derivative, all other SDHI, including the new market entries benzovindiflupyr (II) and fluxapyroxad (I), having an anilide moiety. Isofetamid has been shown to be highly effective against Botrytis cinerea (grey mold) on grape. It is registered for the control of different Botrytis and Sclerotinia spp. on grape, lettuce, rapeseed, low growing berry, and turfgrass on golf courses.
Mode of Action
Isofetamid acts specically on the succinate dehydrogenase (SDH) of Complex II, a key enzyme of the mitochondrial respiratory chain at the crossroads of two metabolic pathways essential to fungal cell life. By inhibiting SDH, Isofetamid impairs energy (ATP) production by the respiratory chain and the synthesis of amino acids, lipids and fatty acids (metabolites essential to cell function) at the Krebs cycle stage.
Advantages of Isofetamid for resistance management
Isofetamid can control numerous isolates with confirmed resistance to other SDHI fungicides, including SdhB H272R and H272Y, which are the two most common field-collected isolates. Research has confirmed that Isofetamid fits the mutated binding pocket of SDHI-resistant fungal isolates (SdhB H272R and H272Y). It is hypothesized that the unique molecular structure of Isofetamid gives the molecule fiexibility at the binding site, allowing Isofetamid to retain efficacy on these mutants. Other SDHI fungicides have a rigid structure, are unable to bind at sites where mutations have occurred, and are therefore ineffective as control options.