Pyraziflumid: Synthesis and Introduction
Synthesis of Pyraziflumid
Pyraziflumid is synthesised using ethyl 4,4,4-trifluoro-3-oxobutanoate as a raw material by chemical reaction. The specific synthesis steps are as follows:
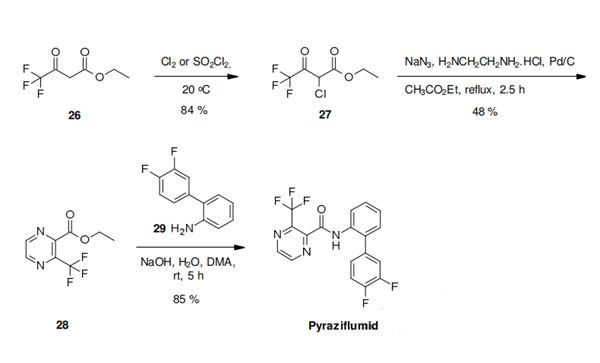
With its unique 2-trifluoromethylpyrazine-3-carboxylic acid, pyraziflumid is only the second complex II inhibitor which contains a six-membered heterocyclic moiety as the carboxylic acid portion. The pyrazine building block 28 is prepared in just two steps from ethyl 4,4,4-trifluoro-3-oxobutanoate (26) in a process which involves chlorination of the β-ketoester in position 2 with chlorine or sulfuryl chloride, followed by a subsequent one-pot transformation with sodium azide, 1,2-ethylenediamine and palladium on charcoal. Hereby the 2- chloroketoester 27 is converted by sodium azide into the corresponding 2-iminoketoester, which undergoes ring closure with 1,2-ethylenediamine to form a dihydropyrazine that is aromatized in situ by the palladium. The ester 28 thus formed can then directly be transformed into pyraziflumid with 2-(3,4-difluorophenyl)aniline (29) under basic conditions.
Introduction of Pyraziflumid
Pyraziflumid is the fourth, and most recent, SDHI presented to the public before 2015. It is currently under development at Nihon Nohyaku. Pyraziflumid is another carboxamide with a biphenylaniline moiety making it closely related to the other SDHI's boscalid (1), bixafen (2) and fluxapyroxad. Therefore, its 2-(3,4-difluorophenyl)aniline can be easily prepared using the Suzuki-Miyaura methodology leading to boscalid's biphenylamine or the Negishi coupling delivering the amine moiety of fluxapyroxad as previously discussed.
Pyraziflumid is a novel succinate dehydrogenase inhibitor (SDHI) fungicide discovered and developed by Nihon Nohyaku Co., Ltd. It exhibits excellent fungicidal activities against a broad range of plant diseases and has a favorable safety profile for the Integrated Pest Management (IPM) program. This compound was found by researching the unique chemical derivatives, 3-(trifluoromethyl)pyrazine-2-carboxamides, and has good biological properties, such as preventive, residual and curative activity, and rain-fastness. Pyraziflumid was registered and launched in Japan in 2018. It was registered in South Korea in 2018 and is now under development in other countries.
Biological Activity
Pyraziflumid showed high antifungal activity based on the inhibition of mycelium growth and spore germination in the agar plate assay using PDA (potato dextrose agar), YBA (yeast extract, Bacto peptone and sodium acetate) and YB (yeast extract and Bacto peptone) media. Its EC50 values were lower than 0.1 mg a.i./L against ascomycetes fungi such as Botrytis cinerea, Cladosporium cucumerinum, Corynespora cassiicola, Didymella bryoniae, Diplocarpon mali, Elsinoë fawcetti, Elsinoë ampelina, Monilinia mali, Pseudocercospora vitis, Sclerotium cepivorum, Sclerotinia sclerotiorum, Venturia inaequalis and Zygophiala jamaicensis. The antifungal activity of the compound was also confirmed against deutromycetes such as Alternaria spp. and Phoma lingam and basidiomycetes such as Rhizoctonia solani and Sclerotium rolfsii. However, pyraziflumid did not show any antifungal activity at 100 mg a.i./L against Colletotrichum spp., Diaporthe citri, Fusarium oxysporum, Penicillium digitatum, Pestalotiopsis menezesiana, Phomopsis fukushii, or Phytophthora infestans. Besides the effect on mycelium growth and spore germination, pyraziflumid exhibits inhibition activity against the apothecium formation of Sclerotinia sclerotiorum.
Safety Properties
Pyraziflumid and its formulated products showed low acute mammalian toxicity and no critical eye or skin irritation. Though the formulation product of pyraziflumid caused mild skin sensitization, it was classified as an out of category in the Globally Harmonized System (GHS). In prolonged exposure studies such as those for carcinogenicity, teratogenicity, reproductive toxicity, and genotoxicity, it was confirmed that pyraziflumid did not adversely affect human health. Acute fish and aquatic invertebrates toxicity of pyraziflumid was low, and it was less bioaccumulative. Consequently, pyraziflumid is considered to be environmentally safe as long as it is used according to its usage standards.


