Isavuconazole: The Antifungal Warrior in the Battle Against Invasive Fungal Diseases
Abstract
Isavuconazole, a second-generation broad-spectrum triazole antifungal agent, has emerged as a potent weapon in the fight against invasive fungal diseases (IFDs). With its unique molecular structure and extensive antifungal spectrum, isavuconazole has shown promising clinical efficacy and favorable safety profiles in treating various life-threatening fungal infections. This review delves into the pharmacological mechanisms, clinical applications, and recent advances of it, highlighting its significance in modern antifungal therapy.
Introduction
Invasive fungal diseases represent a significant public health challenge, particularly among immunocompromised populations. The escalating incidence of these infections, coupled with high mortality rates, underscores the urgent need for effective antifungal agents. Isavuconazole, a novel triazole antifungal drug, has garnered significant attention due to its broad-spectrum activity, favorable pharmacokinetics, and good tolerability.
Pharmacology and Mechanism of Action
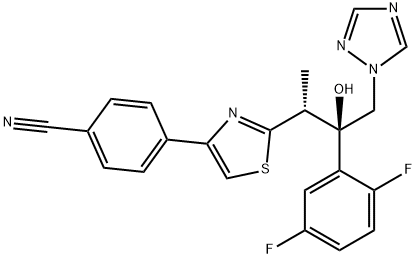
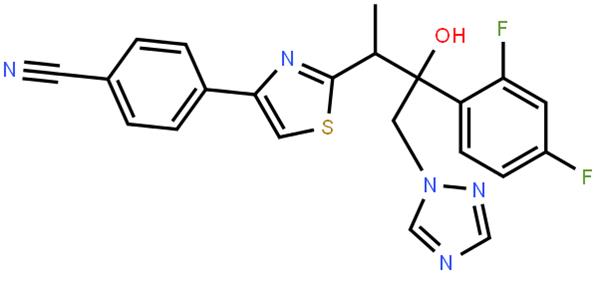
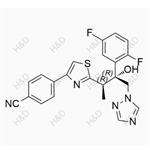
Isavuconazole exerts its antifungal effects by inhibiting the cytochrome P450 (CYP)-mediated 14α-lanosterol demethylase, a crucial enzyme in the ergosterol biosynthesis pathway of fungi. By blocking this enzyme, isavuconazole disrupts the fungal cell membrane structure, leading to membrane dysfunction, increased permeability, and ultimately, cell death. Its unique molecular structure, featuring the [N-(3-acetoxypropyl)-N-methylamino]-carboxymethyl side chain with two chiral centers, enables the triazole ring to bind selectively to the fungal CYP51 protein, conferring a broader antifungal spectrum compared to other triazoles.
Isavuconazole demonstrates potent in vitro and in vivo activity against a wide range of fungi, including molds (such as Aspergillus species), yeasts (Candida species), dimorphic fungi, and even some rare and drug-resistant strains. This broad-spectrum activity makes it an attractive option for treating complex and recalcitrant fungal infections.1
Clinical Efficacy
Isavuconazole was first approved by the US Food and Drug Administration (FDA) in 2015 for the treatment of adult patients with invasive aspergillosis and mucormycosis. Since then, its clinical efficacy has been demonstrated in numerous studies, with favorable outcomes reported across various patient populations and infection types.2
Invasive Aspergillosis
Isavuconazole has shown non-inferiority to voriconazole, the standard of care for invasive aspergillosis, in terms of overall response rates and survival outcomes. Its efficacy has been confirmed in both first-line and salvage therapy settings, particularly in patients with central nervous system involvement, where it demonstrates good cerebrospinal fluid penetration.3
Mucormycosis
For mucormycosis, a highly aggressive and often fatal fungal infection, isavuconazole has demonstrated promising results as salvage therapy in patients refractory to other antifungals. Its use as primary therapy is currently under investigation, with early data suggesting potential benefits.4
Other Fungal Infections
Isavuconazole has also been evaluated for the treatment of other IFDs, including cryptococcosis, candidiasis, and rare fungal infections. While data are limited, initial findings suggest its potential utility in these indications as well.
Safety and Tolerability
Isavuconazole boasts a favorable safety profile, with fewer drug-related adverse events compared to some other antifungal agents. Patients typically tolerate it well, with a low incidence of hepatotoxicity, nephrotoxicity, and other serious adverse reactions. The drug-drug interaction potential is also relatively low, allowing for easier management in complex clinical settings.
Recent Advances and Future Directions
Recent years have seen significant progress in the development and clinical application of isavuconazole. The availability of both oral and intravenous formulations has expanded its therapeutic options, making it suitable for both outpatient and inpatient management. Ongoing research continues to explore its role in novel indications, such as prophylaxis and empiric therapy in high-risk patient populations.
Additionally, studies investigating the optimal dosing strategies and pharmacokinetic/pharmacodynamic relationships of isavuconazole are underway, aiming to further optimize its clinical use. The development of resistance to it, although rare, is also being closely monitored to ensure the long-term effectiveness of this valuable antifungal agent.5
Conclusion
Isavuconazole, with its broad-spectrum antifungal activity, favorable safety profile, and predictable pharmacokinetics, has emerged as a valuable addition to the antifungal armamentarium. Its clinical efficacy in treating various IFDs, particularly invasive aspergillosis and mucormycosis, has been well established. As research continues to unfold, the role of it antifungal therapy is expected to expand further, benefiting an increasing number of patients worldwide.
References:
[1] NATASHA N PETTIT P L C. Isavuconazole: A New Option for the Management of Invasive Fungal Infections.[J]. Annals of Pharmacotherapy, 2015, 49 7. DOI:10.1177/1060028015581679.[2] DUSTIN T WILSON. Role of isavuconazole in the treatment of invasive fungal infections.[J]. Therapeutics and Clinical Risk Management, 2016, 12. DOI:10.2147/TCRM.S90335.
[3] M. SHIRLEY L S. Isavuconazole: A Review in Invasive Aspergillosis and Mucormycosis[J]. Drugs, 2016, 76 1: 1647-1657. DOI:10.1007/s40265-016-0652-6.
[4] ALESSANDRO C PASQUALOTTO L Z G Karoline O Thiele. Novel triazole antifungal drugs: focus on isavuconazole, ravuconazole and albaconazole.[J]. Current opinion in investigational drugs, 2010, 11 2.
[5] WILBY K J. A Review of the Clinical Pharmacokinetics and Pharmacodynamics of Isavuconazole.[J]. European Journal of Drug Metabolism and Pharmacokinetics, 2018, 43 3. DOI:10.1007/s13318-017-0445-7.
Lastest Price from Isavuconazole manufacturers
US $0.00/kg2025-11-21
- CAS:
- 241479-67-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $0.00-0.00/mg2025-05-14
- CAS:
- 241479-67-4
- Min. Order:
- 10mg
- Purity:
- 98%
- Supply Ability:
- 500mg