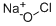Is Sodium Hypochlorite an Acid or Base?
Bleach is another name for Sodium Hypochlorite or NaOCl and is taken as a sodium salt of hypochlorous acid. There is a common misconception of bleach as just one chemical compound; it is a name given to an entire class of chemicals, some oxidizing and reducing agents. One of the bleachs-Sodium hypochlorite (NaOCl), is a solution made from reacting chlorine with a sodium hydroxide solution. These two reactants are the major co-products of most chlor-alkali cells. Sodium hypochlorite, commonly called bleach, has various uses and is an excellent disinfectant/antimicrobial agent.

Sodium hypochlorite is a clear, slightly yellowish solution with a characteristic odor. It has a relative density of 1,1 (5,5% watery solution). As a domestic bleaching agent, it usually contains 5% sodium hypochlorite (with a pH of around 11; irritating). If it is more concentrated, it contains a concentration of 10-15% sodium hypochlorite (with a pH of around 13, it burns and is corrosive).
Sodium hypochlorite is unstable. Chlorine evaporates at a rate of 0,75 grams of active chlorine per day from the solution. Then, heated sodium hypochlorite disintegrates. This also happens when sodium hypochlorite comes in contact with acids, sunlight, certain metals, and poisonous and corrosive gasses, including chlorine gas. Sodium hypochlorite is a strong oxidator and reacts with flammable compounds and reductors. Sodium hypochlorite solution is a weak base that is inflammable.
Sodium hypochlorite (NaOCl) is quite soluble in water, so it quickly dissolves into Na+ and OCl − ions. Na + does not affect the pH of the solution. However, the hypochlorite (OCl −) ion is the conjugate base of hypochlorous acid (HOCl).
HOCl + H2O ⇌ OCl− + H3O +
As the conjugate base of a weak acid, the hypochlorite ion will have an affinity for protons (it acts as a base).
OCl− + H2O ⇌ HOCl + OH−
This equilibrium lies far to the left but has a noticeable effect on the pH of a solution. Hence, Sodium hypochlorite solution is a weak base.
Due to the presence of caustic soda in sodium hypochlorite, the pH of the water is increased. When sodium hypochlorite dissolves in water, two substances form, which play a role in oxidation and disinfection. These are hypochlorous acid (HOCl) and the less active hypochlorite ion (OCl-). The pH of the water determines how much hypochlorous acid is formed. While sodium hypochlorite is used, hydrochloric acid (HCl) lowers the pH. Sulfuric acid (H2SO4) can be an alternative to acetic acid. Less harmful gasses are produced when sulfuric acid is used.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium hypochlorite manufacturers

US $0.00/KG2025-04-21
- CAS:
- 7681-52-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7681-52-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons