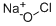What happens when you mix bleach and vinegar?
Bleach and vinegar are common household cleaners that disinfect surfaces, cut through grime, and remove stains. But what you don't know is that mixing these two substances can produce toxic substances that can even be fatal!
Bleach consists primarily of 3-6% sodium hypochlorite (NaClO), also known as liquid bleach. Bleaching powder consists of a mixture of calcium hypochlorite calcium hypochlorite (Ca(ClO)2), calcium hydroxide (slaked lime, Ca(OH)2), and calcium chloride (CaCl2) in variable amounts. Vinegar is a combination of acetic acid and water made by a two-step fermentation process. First, yeast feed on the sugar or starch of any liquid from a plant food such as fruits, whole grains, potatoes, or rice. This liquid ferments into alcohol.
Sodium hypochlorite is made up of sodium, oxygen, and chlorine atoms. When this molecule is mixed with the acetic acid in vinegar or other acid types, it releases chlorine gas. Chlorine gas is hazardous to human health. It's so powerful that Germany used it during World War I as a chemical weapon.

Chlorine bleach contains sodium hypochlorite (NaOCl), but because it's dissolved in water, the chemical exists as hypochlorous acid (HOCl):
NaOCl + H2O ↔ HOCl + Na+ + OH–
Hypochlorous acid is so good at bleaching and disinfecting because it's a strong oxidizer. This also makes it good at participating in undesirable chemical reactions. Mixing bleach with an acid produces chlorine gas. For example, reacting bleach with hydrochloric acid makes water and chlorine:
HOCl + HCl ↔ H2O + Cl2
Vinegar contains diluted acetic acid rather than hydrochloric reaction, but chlorine is still produced:
2HOCl + 2HAc ↔ Cl2 + 2H2O + 2Ac–
You'll know there's a problem if the liquid suddenly smells very strongly of bleach (which is the odor of chlorine). If you see a faint yellowish-green haze, it's a sign of significant chlorine gas production. However, bleach and vinegar dilute, so the gas is generally invisible. Avoid breathing the vapor and immediately leave the area. Return only after the chlorine odor has dissipated. Immediately seek medical attention or call Poison Control if you experience burning or blistering eyes, skin, or mucous membranes or have trouble breathing.
You may like
Related articles And Qustion
Lastest Price from Sodium hypochlorite manufacturers

US $0.00/KG2025-04-21
- CAS:
- 7681-52-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7681-52-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons