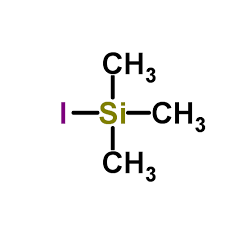
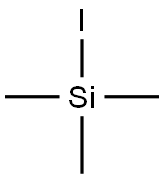Iodotrimethylsilane:an important silanization reagent
Introduction
Iodotrimethylsilane is a versatile organosilicon compound used in organic synthesis. It serves as a source of iodine, allowing for the introduction of iodine atoms into organic molecules. Iodotrimethylsilane acts as a strong electrophile and is stable due to its silicon atom. It is used as a coupling partner in cross-coupling reactions, enabling the formation of carbon-carbon or carbon-heteroatom bonds. Iodotrimethylsilane plays a crucial role in the reductive cleavage of heteroaryl C-halogen bonds, allowing for the synthesis of complex organic molecules. It is also used in the dealkylation of ethers and esters to form alcohols or carboxylic acids. In nucleosidation, iodotrimethylsilane introduces iodine atoms into nucleosides, creating modified nucleosides for biomedical research and drug development. Iodotrimethylsilane is known for its corrosive nature, capable of causing severe burns to the skin and eyes, emphasizing the necessity for proper personal protective equipment such as gloves and safety goggles. Inhalation of vapors or mist may be harmful, necessitating work in well-ventilated areas.
Application
In recent years, the development and application research of safe, non-toxic, environmentally friendly, and highly reactive organic silicon products have been very active. The variety of organic silicon products is becoming increasingly diverse, and popular research areas mainly include modification and application of silanization reagents, ultra-high performance silicone rubber, functionalized silicone oil and high value-added silicone resin, 3D printing materials, organic silicon mild reducing agents, superhydrophobic fabric finishing agents, organic silicon fungicides, silicon containing anti-tumor active drugs, fluorine silicon anti fingerprint transparent coatings, etc. Among them, there are various types of silanization reagents developed, which are widely used in organic synthesis, drug analysis, chromatographic column separation and purification, nanomaterial construction, and other fields. Iodotrimethylsilane is an important silanization reagent with a wide range of applications in the chemical and pharmaceutical fields.
1.Iodotrimethylsilane as a Reactive Ligand for Surface Etching and Passivation of Perovskite Nanocrystals toward Efficient Pure-red to Deep-red LEDs:Resurfacing perovskite nanocrystals (NCs) with tight-binding and conductive ligands to resolve the dynamic ligands-surface interaction is the fundamental issue for their applications in perovskite light-emitting diodes (PeLEDs). Although various types of surface ligands have been proposed, these ligands either exhibit weak Lewis acid/base interactions or need high polar solvents for dissolution and passivation, resulting in a compromise in the efficiency and stability of PeLEDs. Herein, we report a chemically reactive agent (Iodotrimethylsilane, TMIS) to address the trade-off among conductivity, solubility and passivation using all-inorganic CsPbI3NCs. The liquid TMIS ensures good solubility in non-polar solvents and reacts with oleate ligands and produces in situ HI for surface etching and passivation, enabling strong-binding ligands on the NCs surface. We report, as a result, red PeLEDs with an external quantum efficiency (EQE) of ≈23 %, which is 11.2-fold higher than the control, and is among the highest CsPbI3PeLEDs. We further demonstrate the universality of this ligand strategy in the pure bromide system (CsPbBr3), and report EQE of ≈20 % at 640, 652, and 664 nm. This represents the first demonstration of a chemically reactive ligand strategy that applies to different systems and works effectively in red PeLEDs spanning emission from pure-red to deep-red1.
2.Iodotrimethylsilane in nucleosidation procedure:1-O-Acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose (I) was reacted with iodotrimethylsilane (II) and the product, the glycosyl iodide, was coupled with silylated uracil to afford 1-(2,3,5-tri-O-benzoyl-beta-D-ribofuranosyl)uracil (III; 89%), with silyated cytosine to afford, on subsequent acetylation, 1-(2,3,5-tri-O-benzoyl-beta-0D-ribofuranosyl)-4-acetamido-2-(1H)-pyrimidinone (IVb; 81%), and with chloromercuri-N-benzoyl-adenine to afford Va and on subsequent debenzoylation, adenosine (Vb; 49%)2.
3.China is a major user of antibiotics, and antibiotics are closely related to people's lives. The research on antibiotic resistance, degradation mechanisms in the ecological environment, harm caused by antibiotic abuse, impurity and impurity mass spectrometry, and optimization of drug synthesis processes are currently hot topics in research. Exploring optimal process parameters, minimizing by-products, and introducing good reaction reagents or catalysts in the synthesis of antibiotic drugs is a necessary way to improve the quality of raw materials. Iodotrimethylsilane can selectively and efficiently cleave chemical bonds such as ethers, esters, and ketals under relatively mild conditions, exhibiting excellent reaction activity. This makes iodotrimethylsilane playing an increasingly important role in pharmaceutical product development, drug related substance synthesis (especially in the synthesis of cephalosporin antibiotics), and other aspects3.
Synthesis
The synthesis of iodotrimethylsilane mainly includes two preparation methods using hexamethyldisilane and trimethylchlorosilane as starting materials. Wang Junhua et al. used hexamethyldisilane as the starting material, and optimized the synergistic catalysis of iodizing agent and phosphorus to obtain high-purity iodotrimethylsilane. Li Chenglin et al. used anhydrous sodium iodide, anhydrous lithium chloride, and trimethylchlorosilane as starting materials to first prepare crude iodotrimethylsilane, which was then distilled to obtain high-purity iodotrimethylsilane3.
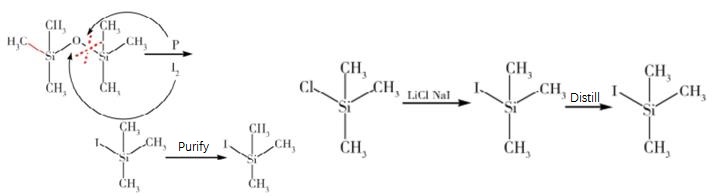
Figure 1 Synthesis of Iodotrimethylsilane
Safety
The compound is known for its corrosive nature, capable of causing severe burns to the skin and eyes, emphasizing the necessity for proper personal protective equipment such as gloves and safety goggles. Inhalation of vapors or mist may be harmful, necessitating work in well-ventilated areas. Iodotrimethylsilane exhibits reactivity and can react violently with water and other substances, demanding careful handling to prevent accidental reactions. Storage recommendations must be followed, keeping the reagent away from incompatible substances. In case of exposure, immediate first aid measures are essential, and users should be aware of the flammable nature of iodotrimethylsilane. Proper waste disposal and adherence to safety guidelines ensure secure use in laboratory settings. It is crucial to consult the material safety data sheet (MSDS) for detailed information on hazards and emergency procedures specific to iodotrimethylsilane, and researchers should be trained to handle it safely in accordance with institutional guidelines.
Reference
1. Zhao F, Duan HW, Li SN, Pan JL, Shen WS, Li SM, Zhang Q, Wang YK, Liao LS. Iodotrimethylsilane as a Reactive Ligand for Surface Etching and Passivation of Perovskite Nanocrystals toward Efficient Pure-red to Deep-red LEDs. Angew Chem Int Ed Engl. 2023 Nov 13;62(46):e202311089.
2. Tocik Z, Earl RA, Beránek J. The use of iodotrimethylsilane in nucleosidation procedure. Nucleic Acids Res. 1980 Oct 24;8(20):4755-61.
3. Wang Junxu, Zhang Le, Duan Lingfeng, et al. Progress in the synthesis of antibiotic drugs involving trimethyliodosilane [J]. Organic Silicon Materials, 2022, 36 (02): 72-76.
Related articles And Qustion
See also
Lastest Price from Iodotrimethylsilane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 16029-98-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons
US $0.00-0.00/KG2025-04-04
- CAS:
- 16029-98-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton