Introduction of Sodium Phosphate, Dibasic
General description
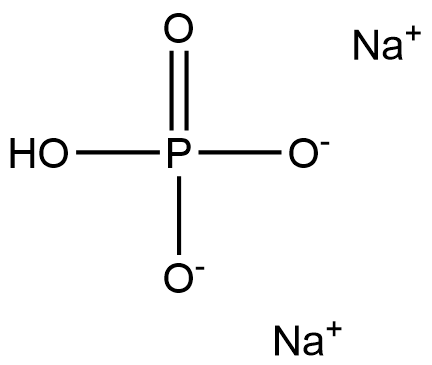
Sodium phosphate, dibasic appears as a colorless to white crystalline solid. Soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used as a fertilizer, in pharmaceuticals, in food processing, and for many other uses. Disodium hydrogen phosphate dodecahydrate is an inorganic substance with the chemical formula Na2HPO4 · 12H2O. It is colorless, translucent crystal or white crystalline powder. It is easily soluble in water and insoluble in ethanol. As a buffer and chelating agent, it is widely used in the field of pharmaceutical preparations. In treatment, sodium dihydrogen phosphate can be used as a mild laxative and for the treatment of hypophosphatemia. Sodium dihydrogen phosphate can also be used in food and as an emulsifier in cheese processing.
Application and Pharmacology
Pharmaceutical preparations, quality improvers, compound fermentation powder, stabilizer for hydrogen peroxide bleaching in printing and dyeing industry, raw materials for manufacturing sodium pyrophosphate and other phosphates, electroplating and tanning.
1.Evaluation of inflammatory cells subsequent to subcutaneous implantation of white mineral trioxide aggregate (WMTA) mixed with disodium hydrogen phosphate (Na2HPO4) in rats.Methods:Forty Wistar rats were used in this study. Polyethylene tubes filled with WMTA mixed with Na2HPO4and WMTA alone and also empty tubes serving as control were implanted into subcutaneous tissue and harvested after 7, 15, 30, and 90 days. Histologic sections were stained with hematoxylin-eosin and observed under a light microscope. Inflammatory reactions were categorized as 0ornone(without inflammatorycells),1ormild(inflammatory cells<25),2ormoderate(25–125 inflammatory cells), and 3 or severe (more than 125 inflammatory cells).Results: WMTA alone provoked a moderate inflammatory reaction after 7 and 15 days, which significantly differed from WMTA mixed with Na2HPO4and the control group, which provoked a mild inflammatory reaction (P< .05). However, there were no significant differences at any period beyond 30 days.Conclusions:This study indicates that adding Na2HPO4to WMTA creates a more biocompatible material than WMTA alone.
2.The melting and crystallization of disodium hydrogen phosphate dodecahydrate were analyzed by means of melting curve,cooling curve and XRD.The products formed by cooling are different at different temperatures,and Na2HPO412H20 was produced by cooling at 40℃and45℃and Na2HP047H2O was produced at 50C.The experiment shows that disodium hydrogen phosphate dodecahydrate will lose water to form Na2HPO47H2O and its solution at 45℃while Na2HPO47H2O will continue to lose water to form a solution when the temperature is higher than 50℃,and the loss of water is reversible. The melting process of disodium hydrogen phosphate dodecahydrate is divided into two stages: physical melting and chemical dehydration. Before water loss, the closer to the critical state of dehydration, the greater the supercooling degree is, and the maximum temperature can reach25.5℃Solution and precipitation were formed after water loss.5mL,10ml and 15ml water were added to each 10g sample to dissolve the precipitation,and the subcooling degree of the obtained solution was tested. which was 9.8℃13.1℃ and 14.4℃,respectively. The lower the concentration of the solution, the greater the subcooling degree.Four different nucleating agents,sodium silicate,diatomite, aluminum nitride and bentonite,were used as nucleating agents and their effects on the it.
Toxicity
Disodium hydrogen phosphate is widely used as an excipient in injectable, oral and topical preparations. Phosphate widely exists in the body and participates in many physiological processes because it is the main anion of intracellular liquid. Most foods contain a considerable amount of phosphate. Except for patients with certain diseases or receiving total nutrition infusion, most people will not suffer from hypophosphatemia (phosphate deficiency). Generally, oral administration of phosphate less than 100mmol per day can treat this disease. About 2 / 3 of the phosphate absorbed is absorbed by the gastrointestinal tract. In essence, almost all phosphate is excreted through urine and the rest is excreted through feces. Excessive phosphate intake (especially through intravenous and rectal administration, or in patients with renal failure) may cause hyperphosphatemia, hypocalcemia or other serious electrolyte disorders. Phosphate can be used as a mild salt laxative by oral or rectal administration. Disodium hydrogen phosphate, as an adjuvant of oral preparations, may cause gastrointestinal dysfunction, including diarrhea, nausea, vomiting and so on. However, when disodium hydrogen phosphate is used as the auxiliary material of pharmaceutical preparations, there are generally no adverse reactions. LD50 (rat, oral): 17g / kg [2] oral maximum dosage 300.00mg; The maximum dosage for eye use was 1.28%; The maximum dosage for ear use was 0.79%; The maximum dosage of nasal cavity was 0.50%; The maximum dosage for local external use is 0.36%; Maximum dosage for injection: 21.30%.
Reference
1.Lotfi M., Vosoughhosseini S. & Saghiri M. A. et al., "Effect of White Mineral Trioxide Aggregate Mixed With Disodium Hydrogen Phosphate on Inflammatory Cells," Journal of Endodontics, Vol.35, No.5(2009), pp.703-705.
2.Hao Ce: Study on modification and application of disodium hydrogen phosphate dodecahydrate phase change energy storage materials: Hebei Agricultural University, 2021.
You may like
Related articles And Qustion
Lastest Price from Sodium Phosphate, Dibasic manufacturers

US $1200.00-1100.00/ton2025-07-31
- CAS:
- 7558-79-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00/KG2025-04-21
- CAS:
- 7558-79-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
![270-82-6 Benzo[c]thiopheneStructureSynthesis](/NewsImg/2024-11-11/6386693836525110707089929.jpg)
![270-82-6 Benzo[c]thiopheneStructureSynthesis](/NewsImg/2022-01-28/6377897911205665289585131.jpg)