Introduction of Loratadine
General description
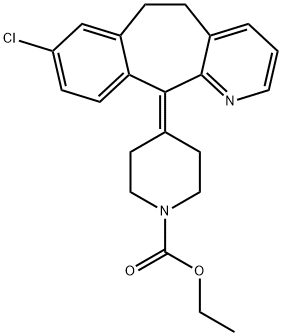
Loratadine is a piperidine histamine H1-receptor antagonist with anti-allergic properties and without sedative effects. Loratadine blocks the H1 histamine receptor and prevents the symptoms that are caused by histamine activity on capillaries, bronchial smooth muscle, and gastrointestinal smooth muscle, including vasodilatation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscle. Loratadine does not cross the blood-brain barrier and does not cause central nervous system effects. Loratadine is a benzocycloheptapyridine that is 6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine substituted by a chloro group at position 8 and a 1-(ethoxycarbonyl)piperidin-4-ylidene group at position 11. It is a H1-receptor antagonist commonly employed in the treatment of allergic disorders. It has a role as a geroprotector, a H1-receptor antagonist, an anti-allergic agent and a cholinergic antagonist. It is an ethyl ester, a N-acylpiperidine, a tertiary carboxamide, an organochlorine compound and a benzocycloheptapyridine. It derives from a desloratadine. Loratadine is a second generation antihistamine used to manage symptoms of allergic rhinitis. A lack of sedative and CNS adverse effects make loratadine, along with other second generation antihistamines, preferable over their 1st generation counterparts in many clinical situations.
Application and Pharmacology
Loratadine,as a second-generation classic anti-allergic drug, can antagonize the binding of histamine and its Hl receptor. Because of its high receptor selectivity, good curative effect, and low side effects, it has become the first-line drug for the treatment of allergic diseases in clinical practice. In addition Loratadine is also an important pharmaceutical and chemical intermediate for the synthesis of the third-generation antihistamines Desloratadine and Rupatadine.
Piperidine antihistamines, a derivative of azatadine, selectively antagonize the effect of peripheral histamine H1 receptor. Its antihistamine effect is fast, strong and lasting. Its effect is stronger than that of astemizole and terfenadine. This product has no sedative effect and no anti muscarinic choline effect. It has no strengthening effect on ethanol. Rapid and good absorption after oral administration. The peak time Tmax of plasma drug concentration was 1.5 hours. The binding rate with plasma protein was 98%. Most of them are metabolized in the liver, and the metabolite decarboxyethoxyloratadine still has antihistamine activity. This product and its metabolites are excreted from urine and feces, T1 / 2 is about 20 hours. This product and its metabolites are not easy to pass through the blood-brain barrier, but can appear in milk.
Good oral absorption, rapid and extensive metabolism in the liver, and excreted by urine and feces. After taking the medicine, the effect is fast, and some patients show the effect within 30 minutes. Tmax is 1.5 ~ 2 h, and the elimination half-life is 8 ~ 14 h. The half-life of the active metabolite decarboxymethylethoxyloratadine (DCL) is 17 ~ 24h. The half-life of the elderly and patients with liver disease may be longer. The binding rate of loratadine to plasma protein was 97% ~ 99%, and DCL was 73% ~ 76%. After 24 hours, about 27% of loratadine was excreted from urine, about 40% was eliminated from urine and 42% was excreted from stool after 10 days. With less milk secretion, it is safe to use drugs during lactation.
Synthesis
The structural characteristics, structure-activity relationships and clinical advantages of loratadine were briefly described in this article, the currently reported synthetic routes of loratadine were systematically analyzed, and after a comprehensive comparison of existing synthetic researches, a synthetic route was determined and its process was optimized in this paper.In this thesis,2-cyano-3-methylpyridine is used as the starting material, and it undergoes Ritter reaction under acidic conditions, and then undergoes alkylation reaction under the catalysis of alkali, and then undergoes phosphorus oxychloride deprotection and Grignard reaction, cyclization reaction, and ethyl chloroformate substitution reaction, to obtain loratadine. The feed ratio of raw materials and reaction conditions such as reaction temperature, catalyst and other factors was systematically investigated and their influence on the total yield of the target compound were explored. The crystallization method of the Ritter reaction product was changed, and the post-treatment process was innovated too; in the cyclizati on reaction step , The production costs was reduced by use of a che ap and low-to xic cyclizati on system, reaction conditions were improved, and the formation of by-products were effectively inhibited. A simple, green and economic synthesis route for loratadine was obtained,with a total yield of31%,which is suitable for scale-up production[1].
Figure 1 The Synthesis route of loratadine
Safety
Montelukast / loratadine (M / L) combination therapy has been widely used to improve the clinical symptoms of patients with allergic rhinitis, but whether M / L combination therapy is better than monotherapy remains controversial[2]. Adverse reactions (1) were less. Occasionally dry mouth, headache, etc. (2) Occasionally, abnormal liver function, jaundice, hepatitis and liver necrosis are seen. Those with impaired liver function should be reduced. (3) Erythema multiforme and systemic anaphylaxis are rare. Contraindications: it is forbidden for those who are allergic to this product. Not recommended for children under 2 years old. Use with caution for pregnant and lactating women.
References
1.Yao Zhongquan: Study on the synthetic process of loratadine: Chengdu University, 2021.
2.Huang Xinmei, Zhang Yanhui, Zheng shameng: Meta analysis of montelukast / loratadine combination therapy on allergic rhinitis, Zhejiang practical medicine, 2017, No. 01, pp. 73-79.
You may like
Related articles And Qustion
Lastest Price from Loratadine manufacturers

US $0.00-0.00/KG2025-09-30
- CAS:
- 79794-75-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $0.00/kg2025-04-25
- CAS:
- 79794-75-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg