Introduction of Edoxaban
General description
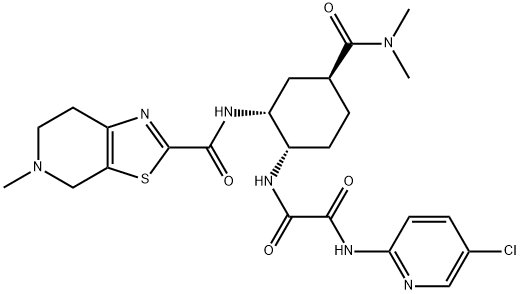
Edoxaban is an orally active inhibitor of coagulation factor Xa (activated factor X) with anticoagulant activity. Edoxaban is administered as Edoxaban tosylate. This agent has an elimination half-life of 9-11 hours and undergoes renal excretion.Edoxaban (1), with chemical name of N - (5-chloropyridine-2-yl) - n '- [(1s, 2R, 4S) - 4 - (n, n-dimethylcarbamoyl)] - 2 - [(5-methyl-4,5,6,7-tetrahydro-1,3-thiazolo [5,4-c] pyridin-2-formamido) cyclohexyl] oxalamide, is a coagulation factor Xa inhibitor developed by Japan's first co stock Society. It was first listed in Japan in April 2011, The trade name is lixina, and the effective component is 1 p-toluenesulfonate monohydrate.Edoxaban is a monocarboxylic acid amide that is used for the treatment of deep vein thrombosis and pulmonary embolism. It has a role as an anticoagulant, an EC 3.4.21.6 (coagulation factor Xa) inhibitor and a platelet aggregation inhibitor. It is a monocarboxylic acid amide, a chloropyridine, a thiazolopyridine and a tertiary amino compound. It is a conjugate base of an edoxaban(1+).
Edoxaban is a selective direct inhibitor of FXa which was developed by Daichi Sankyo Company Limited. It can be used to suppress venous
thromboembolism of the patients who have the surgery with their lower limbs. Edoxaban went on sale in Japan in July, 2004. The market prospect is very broad.
Application
Edoxaban is an oral, small molecule inhibitor of factor Xa which is used as an anticoagulant to decrease the risk of venous thromboses, systemic embolization and stroke in patients with atrial fibrillation, and as treatment of deep vein thrombosis and pulmonary embolism to prevent thrombotic complications. Edoxaban has been linked to a low rate of serum aminotransferase elevations during therapy, but has not been implicated in cases of clinically apparent acute liver injury.
1.Edoxaban is the last direct oral anticoagulant marketed for the prevention of stroke among patients with nonvalvular atrial fibrillation (AF) The primary safety end point will be the incidence of International Society on Thrombosis and Haemostasis-defined major or clinically relevant nonmajor bleeding and the main efficacy end point the composite of cardiovascular death, stroke, systemic embolic events, spontaneous myocardial infarction, and definite stent thrombosis.[1]
2.Low-molecular-weight heparin is the standard treatment for cancer-associated venous thromboembolism. The role of treatment with direct oral anticoagulant agents is unclear.[2] The relative efficacy and safety of edoxaban 60 mg compared with warfarin were independent of different clinical conditions, such as prior stroke, age, risk of falls, renal function, hepatic disease, ischemic heart disease, heart failure, valvular heart disease, or cancer. Data about the effectiveness and safety of edoxaban in real-life patients are scarce, but consistent with those of the pivotal clinical trial.
3.Oral edoxaban was noninferior to subcutaneous dalteparin with respect to the composite outcome of recurrent venous thromboembolism or major bleeding. The rate of recurrent venous thromboembolism was lower but the rate of major bleeding was higher with edoxaban than with dalteparin.
Synthesis
The synthetic routes of (edoxaban,1)and its important intermediates 5-methyl-4,5,6,7 tetrahydrothiazolo [5,4-c] PYRIDINE-2-CARBOXYLIC acid lithium salt (2) and 2 - [(5 chloropyridine-2-yl) amino] - 2-oxoacetate lithium salt (3) were studied. 1 has three chiral centers, and the key to its synthesis is the synthesis of chiral diamine derivatives. (edoxaban,1) can be obtained by condensation of chiral diamine derivatives with intermediates(2) and(3) . The specific compounds can be seen in the figure. This part mainly introduces the steps of important intermediates related to (edoxaban,1). The specific synthesis is shown in the figure:
Identified a suitable industrial synthetic route of Edoxaban. Edoxaban consists of three main intermediates. The (S) -3- cyclohexen-1-carboxylic acid is the starting material, After the addition of iodine, esterification, nucleophilic substitution reaction, palladium carbon reduction, amino protection, Walden inversion reaction, ester and amide bond hydrolysis reaction, we get the (1R, 2S, 5S)-2-azido-S-[(dimethylamino) carbonyl]-cyclohexyl carbamate. Eventually, after 17-step reactions, we get the final product Edoxaban. The route is suitable for industrial production because it has a shorter reaction period, lower raw material prices, higher product yields. After the Optimization of related technology, the total yield was raised to 1.69% from 0.88%.[3,4]
Figure the synthesis route of Edoxaban
Toxicity
In the ENGAGE AF–TIMI 48 trial, edoxaban 60 mg was noninferior to warfarin for the prevention of stroke or systemic embolism, but significantly reduced the risk of bleeding, major adverse cardiac events and death from cardiovascular causes. The relative efficacy and safety of edoxaban 60 mg compared with warfarin were independent of different clinical conditions, such as prior stroke, age, risk of falls, renal function, hepatic disease, ischemic heart disease, heart failure, valvular heart disease, or cancer. Data about the effectiveness and safety of edoxaban in real-life patients are scarce, but consistent with those of the pivotal clinical trial. Edoxaban seems a cost-effective alternative to warfarin among AF patients with moderate to high thromboembolic risk. [5]
Reference
1.Raskob G. E., van Es N. & Verhamme P. et al., "Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism," New England Journal of Medicine, Vol.378, No.7(2018), pp.615-624.
2.Kato E. T., Giugliano R. P. & Ruff C. T. et al., "Efficacy and Safety of Edoxaban in Elderly Patients With Atrial Fibrillation in the ENGAGE AF–TIMI 48 Trial," Journal of the American Heart Association, Vol.5, No.5(2016).
3.Linyu P., Bin L. & Fuli Z., "Graphical Synthetic Routes of Edoxaban," Chinese Journal of Pharmaceuticals, Vol.44, No.11(2013).
4.Yin Xinhao: synthesis of edoxaban and its related substances: Beijing University of chemical technology, 2015.
5.Raskob G. E., van Es N. & Verhamme P. et al., "Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism," New England Journal of Medicine, Vol.378, No.7(2018), pp.615-624.
You may like
See also
Lastest Price from edoxaban manufacturers

US $0.10/KG2025-09-14
- CAS:
- 480449-70-5
- Min. Order:
- 1KG
- Purity:
- 99%+
- Supply Ability:
- 2000 kgs

US $0.00/KG2025-04-21
- CAS:
- 480449-70-5
- Min. Order:
- 10g
- Purity:
- 99%min
- Supply Ability:
- 10kg