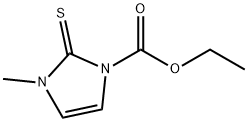
Introduction of carbimazole
General description
Carbimazole is a member of the class of imidazoles that is methimazole in which the nitrogen bearing a hydrogen is converted into its ethoxycarbonyl derivative. A prodrug for methimazol, carbimazole is used for the treatment of hyperthyroidism. It has a role as a prodrug and an antithyroid drug. It is a carbamate ester and a member of 1,3-dihydroimidazole-2-thiones.(Carbimazol;CarbiMazole;3-Methyl-2-thioxo-2,3-dihydro-imidazol-1-carbonsaeure-aethylester;2,3-Dihydro-3-methyl-2-thioxo-1H-imidazole-1-carboxylic acid ethyl ester)
An imidazole antithyroid agent. Carbimazole is metabolized to methimazole, which is responsible for the antithyroid activity. An imidazole antithyroid agent. Carbimazole is metabolized to METHIMAZOLE, which is responsible for the antithyroid activity. White or off white crystalline powder. Melting point 122-125 ℃. At 20 ℃, the product can be dissolved in 500 parts of water, 50 parts of ethanol, 330 parts of ether, 3 parts of chloroform and 17 parts of acetone. It's very smelly and bitter.
Application
Carbimazole tablets are suitable for various types of hyperthyroidism, especially for: 1. Patients with mild to moderate goiter; 2. Adolescents, children and elderly patients; 3. Those who relapse after thyroid surgery and are not suitable for radiation I therapy; 4. Preparation before operation; 5. As adjuvant therapy of I radiotherapy.It is an antithyroid drug and belongs to mercaptoimidazole class with tabazole. Carbimazole is a precursor of tabazole, which is transformed into tabazole in vivo. Its effect and adverse reactions are similar to tabazole, with slow effect and longer maintenance time than tabazole.
Synthesis
It is obtained from chloroacetaldehyde diethanol by amination, cyclization and condensation. 1. Amination add chloroacetaldehyde diethanol, methylamine and methanol into a pressure cooker to react to obtain 2-methylaminoacetaldehyde diethanol. 2. Cyclization: heat 2-methyl chemicalbook aminoacetaldehyde diethanol with hydrochloric acid and potassium thiocyanate to obtain 1-methyl-2-mercaptoimidazole. 3. Condensation: 1-methyl-2-mercaptoimidazole was condensed into pyridine, and ethyl chloroformate was added dropwise to obtain carbimazole.
Figure the synthesis route of Carbimazole
Ethyl chloroformate (0.23 ml, 2.43 mmol) was added through a syringe to a freshly distilled dry THF (30 ml) solution of 1-Methylimidazole (0.19 ml, 2.43 mmol) cooled (0 ℃). The mixture was stirred for 3 hours and then cooled to 0 ℃. After adding triethylamine (0.34 ml, 2.43 mmol), bring the solution to room temperature (25 ℃). After stirring at this temperature for 3 hours, elemental sulfur (0chemicalbook.08g, 2.60mmol) was added. The resulting mixture was stirred for 24 hours and then filtered through a diatomite pad. Evaporate the solvent under reduced pressure to obtain a gray solid. The compound was extracted with chloroform and filtered through diatomite. The desired compound was obtained from the filtrate as a white solid, which was further purified by column chromatography. Carbimazole was obtained as a white crystalline solid using ethyl acetate / petroleum ether (1:1.5) as eluent. Yield: 0.44g (97%)
Toxicity
Carbimazole (CBZ), remain one of the mainstays of treatment for Graves’ disease worldwide and can also be used for the treatment of other causes of hyperthyroidism, such as toxic nodular goiter. The overall incidence of hepatotoxicity with any antithyroid medication is < 0.5%, but published evidence now shows an increased risk of serious liver injury associated with one of the thionamides, PTU, with a higher prevalence in the pediatric population[1]. Because of this, CBZ and its active metabolite, MMI, are now the preferred antithyroid drugs except in select
circumstances. Aches started within the first week of treatment with 30 mg carbimazole daily, continued on a reduced dose of carbimazole (10 mg, not 20 mg as we stated), and disappeared rapidly after transfer to an equivalent dose of propylthiouracil (100 mg). The second patient was taking 30 mg carbimazole for 3 months while abroad and might have been expected to have become hypothyroid.The data are more serious in the pediatric population with the morbidity and mortality being higher compared with adults. Hepatotoxicity can appear at any time during therapy and there is no clear evidence that monitoring will diagnose hepatotoxicity early and prevent severe complications.
Reference
1. "JULIA G. BODMER,".
2.Akmal A. & Kung J., "Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity," Expert Opinion on Drug Safety, Vol.13, No.10(2014), pp.1397-1406.
You may like
Lastest Price from Carbimazole manufacturers

US $0.00-0.00/KG2025-05-19
- CAS:
- 22232-54-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 22232-54-8
- Min. Order:
- 1KG
- Purity:
- 99%min; BP
- Supply Ability:
- 500kg