Indole Synthesis: Methods and Mechanisms
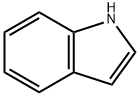
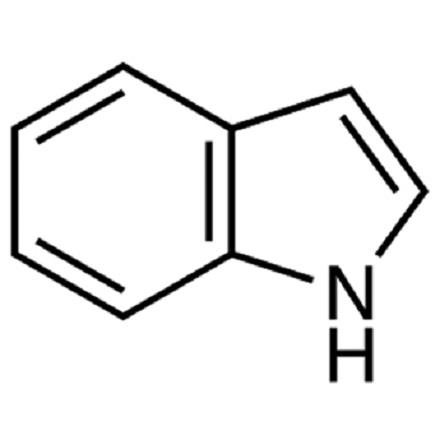
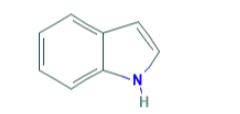
Indole is a benzo[b]pyrrole, a heterocyclic compound formed by the fusion of a benzene ring to the 2,3 positions of a pyrrole nucleus. The term "indole" originates from India, where the heterocycle was first isolated from the blue dye "Indigo" in the sixteenth century. Adolf Baeyer isolated indole in 1886 through the pyrolysis of oxindole with zinc dust. Commercially, indole is produced from coal tar and is the most widely distributed heterocyclic compound, integral to thousands of naturally occurring alkaloids, drugs, and other compounds.
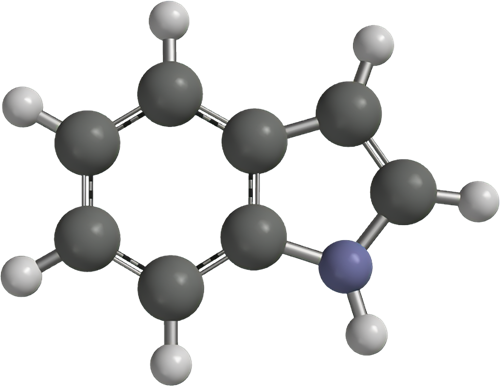
Synthesis of Indole
1. Fischer Indole Synthesis
The Fischer Indole Synthesis, discovered by Emil Fischer in 1983, is the most extensively used method for preparing indoles. It involves the cyclization of arylhydrazones under heating conditions in the presence of protic or Lewis acids. The reaction produces 2,3-disubstituted products, with unsymmetrical ketones potentially yielding a mixture of indoles.
2. Madelung Synthesis
The Madelung Synthesis is a base-catalyzed cyclization of 2-(acylamino)-toluenes under harsh conditions. It is limited to the synthesis of simple indoles, such as 2-methyl indoles, without sensitive groups.
3. Reissert Synthesis
The Reissert Indole Synthesis is a multistep reaction that includes the base-catalyzed condensation of o-nitrotoluene with oxalic ester, reduction of the nitro group to an amino group, cyclization to indole-2-carboxylic acid, and decarboxylation.
4. Bartoli Indole Synthesis
An efficient and practical approach for indole synthesis, where ortho-substituted nitrobenzenes react with vinyl magnesium bromide to yield 7-substituted indoles.
5. Nenitzescu Synthesis
This reaction provides a direct route for the synthesis of 5-hydroxyindoles through the condensation of substituted 1,4-benzoquinone with β-amino-substituted α, β-unsaturated carbonyl compounds, leading to ring closure and formation of 5-hydroxyindole.
6. Bischler Indole Synthesis
Involves the acidic treatment of 2-arylamino-ketones to induce electrophilic cyclization onto the aromatic ring, often resulting in product mixtures due to rearrangements.
Mechanisms
A modern variant of the Madelung reaction is performed under milder conditions using alkyllithiums as bases, allowing the synthesis of 2-substituted indoles with sensitive groups.
You may like
Related articles And Qustion
Lastest Price from Indole manufacturers

US $20.00/kg2025-04-21
- CAS:
- 120-72-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 120-72-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg