Indole:Chemical Properties and Production
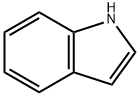
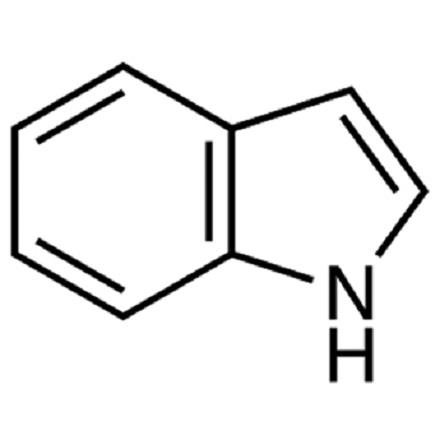
Indole [120-72-9], 1-benzo[b]pyrrole, C8H7N, was discovered by A. v. BAEYER and C. A. KNOP in 1866 as the basic structure of the natural dye, indigo, from which it was obtained. In 1910, R. WEISSGERBER found indole in coal tar.
Physical Properties
Indole, Mr 117.15, mp 52.5 ℃, bp 254.7 ℃ (101.3 kPa), d 1.22 g/cm3 (18 ℃), forms colorless, shiny flakes with a slight jasmine aroma. It is readily soluble in ethanol, diethyl ether, and benzene, soluble in hot water, slightly soluble in cold water, and volatile in steam. The heat of combustion is 3.650 kJ/kg (25 ℃), the enthalpy of vaporization 597.5 kJ/ kg (10.3 – 27.4 ℃), and the dipole moment 2.11 D (benzene).
Chemical Properties
As a secondary amine, indole has a hydrogen atom which can be substituted by alkali metals. Oxidation leads to indigo, mild hydrogenation to 2,3-dihydroindole (indoline). Diindole, triindole, and resinification products are obtained upon treatment with acids.
Production
High-temperature coal tar contains on average just under 0.2 % indole. In coal tar distillation, the indole is concentrated in a biphenyl – indole fraction which boils between 245 and 255 ℃. Following extraction of phenols and bases, the indole is isolated from this fraction and separated from the other major component biphenyl, whose boiling point is only 0.3 ℃ higher. This is achieved by melting with potassium hydroxide to give the potassium salt of indole, by azeotropic distillation with diethylene glycol, or by extraction with selective solvents (e.g., glycols, aqueous dimethyl sulfoxide, or monoethanolamine). The crude indole can then be purified by crystallization from aliphatic hydrocarbon solvents.
In addition to its recovery from coal tar, indole is also synthesized in technical quantities. Methods used include Madelung synthesis of formyltoluidine (from o-toluidine and formic acid), followed by cyclization, dehydrogenating cyclization of 2-ethylaniline, cyclocondensation of aniline and ethylene glycol in the liquid or gas phase, and cyclization of 2- (2-nitrophenyl)ethanol.
Related articles And Qustion
See also
Lastest Price from Indole manufacturers

US $20.00/kg2025-04-21
- CAS:
- 120-72-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 120-72-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg