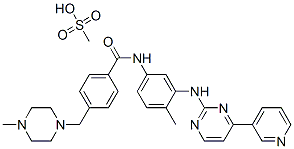
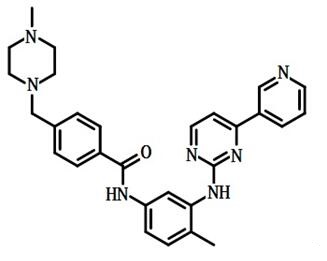
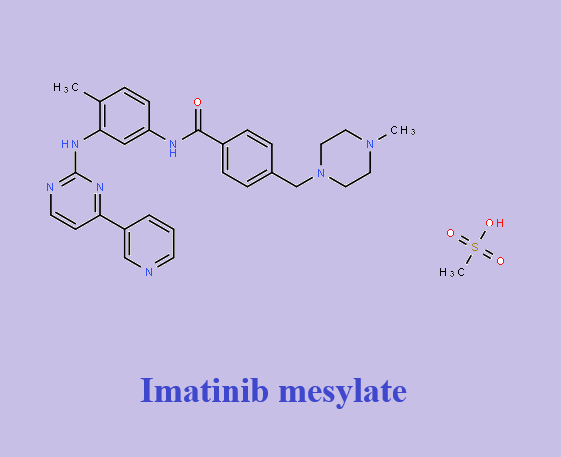
Imatinib mesylate: Indications, Mechanism of Action and Side Effects
Indications
Imatinib mesylate is an oral tyrosine kinase inhibitor used to treat chronic granulocytic leukaemia and certain types of cancer. Current FDA-approved indications include:
First-line adult and pediatric Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase (Ph+ CML CP)
Philadelphia chromosome-positive chronic myelogenous leukemia in blast crisis (Ph+ CML BC)
Philadelphia chromosome-positive chronic myelogenous leukemia in accelerated phase (Ph+ CML AP)
Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase after failure of interferon-alpha therapy
Adult relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL)
First-line pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy
Adult myelodysplastic (MDS)/myeloproliferative (MPD) diseases associated with platelet-derived growth factor receptor gene re-arrangements
Adult aggressive systemic mastocytosis (ASM) without D816V c-Kit mutations (or unknown c-Kit mutational status)
Adult hypereosinophilic syndrome (HES) or chronic eosinophilic leukemia (CEL) with FIP1L1-PDGFR-alpha fusion kinase and for patients with hypereosinophilic syndrome and/or chronic eosinophilic leukemia who are FIP1L1-PDGFR-alpha fusion kinase negative or unknown
Adult unresectable, recurrent, and/or metastatic dermatofibrosarcoma protuberans (DFSP)
Kit positive unresectable and/or metastatic malignant gastrointestinal stromal tumors (GIST)
Adult adjuvant treatment following resection of Kit positive GIST
Mechanism of Action
Imatinib mesylate is a protein tyrosine kinase inhibitor that inhibits bcr-abl tyrosine kinase, a constitutively abnormal tyrosine kinase produced by abnormalities of the Philadelphia chromosome in chronic myeloid leukaemia (CML). It inhibits proliferation and induces apoptosis in bcr-abl-positive cell lines as well as in fresh leukaemia cells from Philadelphia chromosome-positive chronic myeloid leukaemia.Imatinib mesylate blocks the constitutive action of protein tyrosine kinases by acting as a competitive inhibitor of the ABL ATP-binding cleft and inducing apoptosis in leukaemia cells.
Side Effects
More common
Oedema
Nausea
Vomiting
Muscle cramps
Musculoskeletal pain
Diarrhoea
Skin rash
Fatigue
Headaches
Joint pain
Abdominal pain
Nasopharyngitis
Bleeding
Dyspepsia
Cough
Sore throat
Upper Respiratory Tract Infection
Weight gain
Insomnia
Depression
Influenza
Thrombocytopenia
Blurred vision
Decreased white blood cells
Less common
Back pain
bad, unusual, or unpleasant (after) taste
change in taste
watering of the eyes
hypopigmentation
lichenoid reactions
pityriasiform eruptions
pityriasis rosea
psoriasis
reactivation or induction of porphyria cutanea tarda
neutrophilic eccrine hidradenitis
Sweet's syndrome
erythema nodosum
References:
[1] YEGYU SUNG Eujin C Min Hee Kim. Successful hyaluronic acid filler injection in a chronic myeloid leukemia patient taking imatinib mesylate.[J]. Journal of Cosmetic and Laser Therapy, 2019, 21 4. DOI:10.1080/14764172.2018.1525745.[2] SCHEINFELD N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate.[J]. Journal of Drugs in Dermatology, 2006, 5 3.
You may like
Related articles And Qustion
Lastest Price from Imatinib mesylate manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 220127-57-1
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 220127-57-1
- Min. Order:
- 1KG
- Purity:
- 98%-102% HPLC
- Supply Ability:
- 500KGS