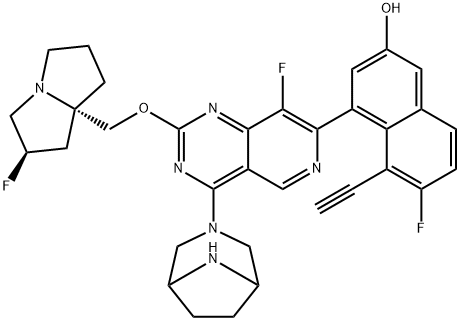
Identification of MRTX1133, a Noncovalent, Potent, and Selective KRASG12D Inhibitor
Mutant KRAS has been recognized as an attractive drug target for the treatment of a number of cancers for many decades.KRASG12D, the most common oncogenic KRAS mutation, is a promising target for the treatment of solid tumors. However, when compared to KRASG12C, selective inhibition of KRASG12D presents a significant challenge due to the requirement of inhibitors to bind KRASG12D with high enough affinity to obviate the need for covalent interactions with the mutant KRAS protein.
MRTX1133, discovered through extensive structure-based activity improvement, is a potent, selective, non-covalent KRASG12D inhibitor with picomolar binding affinity, single-digit nanomolar activity in cellular assays, and significant in vivo efficacy in tumor models carrying KRASG12D mutations.
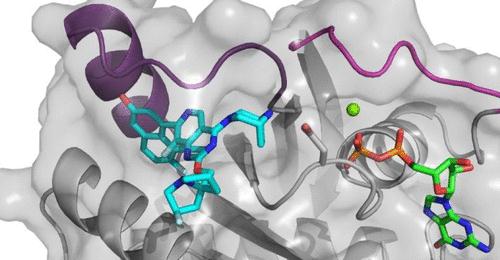
MRTX1133 optimally fills the switch II pocket and extends three substituents to favorably interact with the protein, resulting in an estimated KD against KRASG12D of 0.2 pM. AlphaLISA data confirmed that binding of the inhibitor prevented SOS1-catalyzed nucleotide exchange and/or formation of the KRASG12D/GTP/RAF1 complex, thereby inhibiting mutant KRAS-dependent signal transduction.
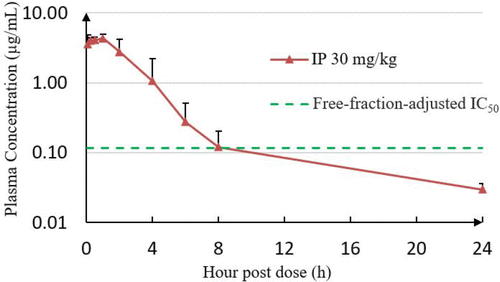
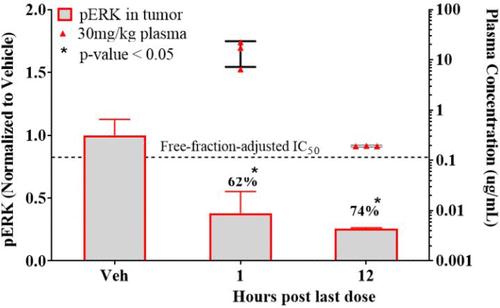
Intraperitoneal (IP) administration of MRTX1133 at 30 mg/kg in CD-1 mice resulted in sustained plasma exposure exceeding the free-fraction-adjusted pERK IC50 value in the KRASG12D mutant Panc 04.03 cell line for approximately 8h.
The ability to modulate KRAS-dependent ERK phosphorylation in the Panc 04.03 xenograft tumor model at 30 mg/kg BID (IP) and observed 62% and 74% inhibition of pERK signal at 1 and 12h after the second dose, respectively.
An antitumor efficacy study in this model resulted in MRTX1133 dose-dependent antitumor activity with 94% growth inhibition observed at 3 mg/kg BID (IP) and tumor regressions of −62% and −73% observed at 10 and 30 mg/kg BID (IP), respectively.
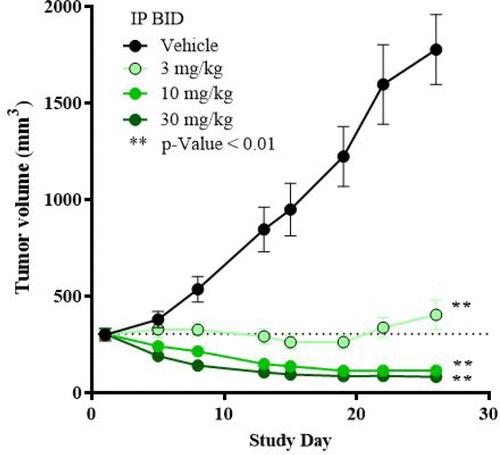
Through extensive structure-based drug design, MRTX1133 was identified as a noncovalent, potent, and selective inhibitor of KRASG12D. MRTX1133 suppresses KRASG12D signaling in cells and in vivo, and its antitumor benefit was demonstrated in a murine animal model. The optimization process was facilitated by high-resolution X-ray crystal structures. In-depth binding mode analysis derived from cocrystal structures allowed the optimization of lipophilic contact of the inhibitor in the binding pocket and the identification of nonclassical hydrogen bonding and ion pair interactions, ultimately increasing selective binding affinity for KRASG12D by more than 1,000,000-fold relative to the initial hit 5B. MRTX1133 binds to the switch II pocket and inhibits the protein–protein interactions necessary for activation of the KRAS pathway. MRTX1133 not only possesses single-digit nM potency in a cellular proliferation assay, but also demonstrates tumor regressions in the Panc 04.03 xenograft model.