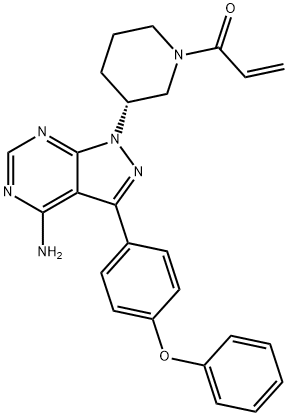
Ibrutinib: Uses, Mechanism of action and Side effects
Uses of Ibrutinib
Ibrutinib is an oral, potent, selective and irreversible small molecule inhibitor of Btk. It is a prescription drug with the trade name IMBRUVICA®. It is approved for the treatment of adult patients with chronic lymphocytic leukaemia (CLL) who have received at least one prior therapy. It is also approved for adult patients with relapsed/refractory sleeve cell lymphoma. It is not known if IMBRUVICA® is safe and effective in children under 1 year of age.
Mechanism of action
Ibrutinib is an orally administered, highly potent, selective, and irreversible small-molecule inhibitor of Btk. It forms a covalent bond with a cysteine residue (CYS-481) at the active site of Btk, leading to inhibition of Btk enzymatic activity. Ibrutinib also abrogates the full activation of Btk by inhibiting its autophosphorylation at Tyr-223. This inhibition prevents downstream activation of the BCR pathway and subsequently blocks cell growth, proliferation, and survival of malignant B cells.
Side effects
The most common non-haematological adverse events with Ibrutinib included diarrhoea, bruising, upper respiratory tract infection, hyperuricaemia, fatigue, musculoskeletal pain, rash, fever, elevated serum creatinine, peripheral oedema, constipation, arthralgia, stomatitis, nausea, sinusitis and dizziness. Serious adverse reactions were infection (pneumonia), hypertension, sinusitis, skin infection, dehydration, musculoskeletal pain and atrial fibrillation. The most common haematological adverse events (20% or more of any level of severity) included thrombocytopenia, neutropenia and anaemia. Serious adverse reactions that may occur include cardiac problems, haemorrhage, hypertension, reduced blood counts, second primary cancers and tumour lysis syndrome (TLS).
Drug and food interactions
Ibrutinib is predominantly metabolized by CYP3A4. Co-administration of ketoconazole, a strong CYP3A4 inhibitor, increased Cmax (the maximum concentration of a drug after its administration) and AUC of ibrutinib by 29-fold and 24-fold, respectively, in healthy volunteers. Consequently, it is advised to avoid concomitant administration of ibrutinib with moderate or strong inhibitors of CYP3A4. If a moderate CYP3A4 inhibitor must be utilized, the manufacturer recommends reducing the dose of ibrutinib to 140 mg/day. If short-term therapy (seven days or less) with a strong CYP3A4 inhibitor (i.e., ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin) is necessary, the clinician may carefully consider interrupting ibrutinib therapy during the duration of inhibitor usage based on risk versus benefit. Chronic therapy with strong CYP3A4 inhibitors should be avoided. Patients should be advised to avoid grapefruit and Seville oranges during ibrutinib therapy, as these contain moderate inhibitors of CYP3A4.
Co-administration of ibrutinib with strong inducers of CYP3A4 decreases ibrutinib plasma concentrations by nearly 10-fold. Therapy with strong CYP3A inducers (i.e., carbamazepine, rifampin, phenytoin, St. John’s wort) should be avoided. Alternative agents with lower CYP3A4 induction properties should be considered.
You may like
Related articles And Qustion
See also
Lastest Price from Ibrutinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 936563-96-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/g/Bag2025-04-21
- CAS:
- 936563-96-1
- Min. Order:
- 25g
- Purity:
- 97%-103%
- Supply Ability:
- 10KGS