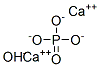Hydroxyapatite: A Key Material in Chemistry and Biomedical Applications
Hydroxyapatite (HA) is a stable and biocompatible calcium phosphate with low solubility being used for various applications such as tooth remineralisation, reduction of tooth sensitivity, oral biofilm control, and tooth whitening. Clinical data on these products is limited with varied results; additionally, the effectiveness of these apatite compounds versus fluoride, which has conventionally been used in toothpaste, has not been established.

Biomedical Applications
Hydroxylapatite is a hard, crystalline mineral composed of calcium, phosphate, and hydroxide ions that is the major constituent of mature bone. As it is an inorganic salt found in normal tissue, it has excellent biocompatibility. Hydroxylapatite has been used for more than 15 years as a reconstructive agent in orthopedic surgery, so has been well studied. It is formulated as microspheres that form a scaffold to which fibroblasts anchor and produce new collagen.[1]
Hydroxyapatite (HA) is a bioactive and non-toxic ceramic that has a close analogy to the inorganic portion of human teeth and bone. It is arranged in a typical lattice structure with the chemical formulation of (Ca10(PO4)6(OH)2). Depending on the manufacturing technique employed, various synthetic apatites are produced today. HA used for biomedical applications is chemically prepared to achieve tailored properties such as chemical purity, crystal morphology, and crystal size. HA’s bioactive, non-toxic, and osteoconductive properties mean it can form direct chemical bonds with living tissues. Thus, HA is a bioceramic that is widely used as an implantable material in dentistry, maxillofacial and orthopaedic surgery to repair bone defects and as a coating material for metallic implants.[2]
References
[1] Jamison E. Strahan, Joel L. Cohen,Chapter 4 - Fillers. Tung,In The Requisites in Dermatology, Cosmetic Dermatology, W.B. Saunders, 2009, Pages 59-79,ISBN 9780702031434.
[2] Lijie Chen.“Hydroxyapatite in Oral Care Products-A Review.”2021.
You may like
Related articles And Qustion
See also
Lastest Price from Hydroxyapatite manufacturers

US $0.00/kg2025-04-30
- CAS:
- 1306-06-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg
US $10.00/KG2025-04-21
- CAS:
- 1306-06-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt