S-Adenosyl-L-methionine: A Comprehensive Overview
Introduction
S-Adenosyl-L-methionine (SAMe) is a crucial compound in biochemistry and molecular biology, recognized for its pivotal role in methylation processes. As the primary methyl donor in a variety of biochemical reactions, SAMe facilitates the transfer of methyl groups, which is essential for the proper functioning of numerous physiological processes. This unique ability makes it indispensable in the regulation of gene expression, protein function, and lipid metabolism. The breadth of its influence spans from DNA and RNA methylation to neurotransmitter synthesis and detoxification pathways. Due to its extensive involvement in critical biological functions, SAMe has become a focal point of research, particularly within the chemical and biochemical fields. Scientists are continually uncovering new applications and mechanisms of action, highlighting SAMe's potential in therapeutic interventions and its significance in maintaining cellular homeostasis. The growing body of research underscores its importance, making it a subject of great interest for professionals aiming to leverage its benefits in both clinical and laboratory settings.

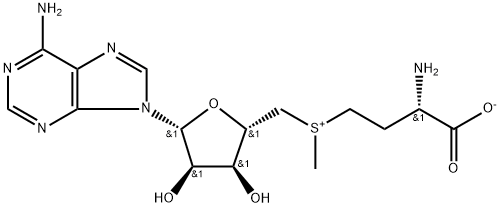
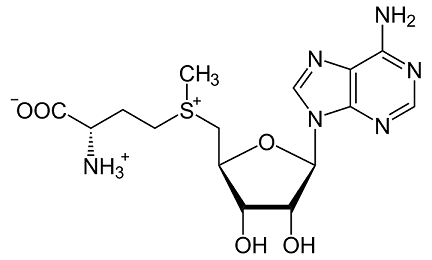
Figure 1 Characteristics of S-Adenosyl-L-methionine
Synthesis of S-Adenosyl-L-methionine
SAMe is synthesized through a reaction between methionine and adenosine triphosphate (ATP). The process, catalyzed by methionine adenosyltransferase (MAT), is a two-step mechanism. Initially, methionine and ATP form an intermediate complex. This complex then rearranges to produce SAMe and inorganic pyrophosphate. The enzymatic process ensures the high specificity and efficiency required for SAMe synthesis. The structural integrity of SAMe is crucial, given its role as a methyl group donor. The methyl group attached to the sulfur atom in SAMe is readily transferable, making it highly reactive and essential in various methylation reactions.
Key Components of S-Adenosyl-L-methionine
SAMe consists of three primary components:
Adenosine: Derived from ATP, adenosine is a nucleoside composed of adenine attached to a ribose sugar. It plays a crucial role in SAMe's structure, contributing to its stability and functionality.
Methionine: An essential amino acid, methionine provides the sulfur atom that is central to SAMe’s methylation capability. The presence of methionine ensures that SAMe can participate in transmethylation processes.
Methyl Group: The transferable methyl group (-CH3) is what makes SAMe a versatile methyl donor. This group is critical in numerous biochemical processes, including DNA methylation, protein methylation, and the synthesis of neurotransmitters.
Applications of S-Adenosyl-L-methionine
SAMe's role as a methyl donor places it at the heart of many biochemical processes. Some of its notable applications include:
Methylation Reactions: SAMe is the primary methyl donor in the methylation of DNA, RNA, proteins, and lipids. This process is crucial for regulating gene expression, maintaining genome integrity, and modulating protein function.
Transsulfuration Pathway: SAMe is a key intermediate in the transsulfuration pathway, which converts methionine to cysteine, another essential amino acid. This pathway is critical for maintaining cellular redox balance and producing glutathione, a major antioxidant.
Neurotransmitter Synthesis: SAMe is involved in the synthesis of neurotransmitters such as serotonin, dopamine, and norepinephrine. This has implications for mood regulation and has led to SAMe being explored as a treatment for depression and other mood disorders.
Storage and Stability of S-Adenosyl-L-methionine
SAMe is sensitive to environmental conditions, making its storage and handling critical for maintaining its efficacy. Key considerations for SAMe storage include:
Temperature: SAMe should be stored at low temperatures, preferably between 2°C to 8°C (35.6°F to 46.4°F), to prevent degradation. Higher temperatures can lead to the breakdown of the compound, reducing its effectiveness.
Light: Exposure to light can also degrade SAMe. It should be stored in dark or opaque containers to minimize light exposure. This helps maintain its stability and prolongs its shelf life.
pH: The stability of SAMe is pH-dependent. It is most stable in acidic conditions (pH 3-5). Alkaline conditions can accelerate its decomposition, so pH control is vital during storage and formulation.
References
[1]Baldessarini R J. Neuropharmacology of S-adenosyl-L-methionine[J]. The American journal of medicine, 1987, 83(5): 95-103.
[2]Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence[J]. The American journal of clinical nutrition, 2002, 76(5): 1158S-1161S.
Related articles And Qustion
Lastest Price from S-Adenosyl-L-methionine manufacturers

US $1.00-4.00/KG2025-09-12
- CAS:
- 29908-03-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $0.00/Kg/Bag2025-04-21
- CAS:
- 29908-03-0
- Min. Order:
- 1KG
- Purity:
- 95%-105%
- Supply Ability:
- 100KGS