How to synthesize nanostructured Magnesium oxide?
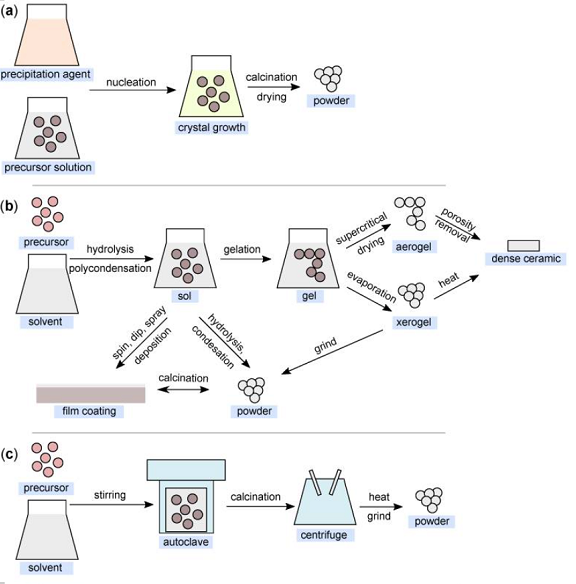
Several approaches can be used for the synthesis of nanostructured MgO. In the following, insights from the most commonly used chemical and biological synthesis methods are presented (bottom-up approach). Specifically, these include sol-gel, solve-/hydrothermal, combustion, co-precipitation, and so-called green synthesis.
Co-Precipitation
This method is also widely used for the synthesis of nanoparticles. It is based on the principle of precipitation and very often involves liquid-phase synthesis and, less often, vapor-phase synthesis. Sodium hydroxide is commonly used as the precipitating agent. The basic principle is the homogenization of the precipitation reaction involving two processes—nucleation and nuclei growth. Generally, three principles are considered: (i) single nucleation and uniform growth by diffusion; (ii) nucleation, growth, and aggregation of smaller subunits; and (iii) multiple nucleations and Ostwald ripening growth. The critical solute concentration that initiates the process plays a significant role in the classical process, with solute diffusion on the surface causing growth. Furthermore, it is necessary to separate these two processes. The resulting precipitate is then washed and dried.
Mashad et al. prepared MgO particles using the co-precipitation method by observing the effect of different reaction conditions, such as temperature, pH, and the molar ratio of precursor (magnesium nitrate). Using the procedure presented in their work, they formed nanoparticles and nanorods with a relatively high specific surface area (nanoparticles 231 m2/g, nanorods 176 m2/g) and particle size of 50 nm. The results show that template polyethene glycol (PEG) and pH affect particle morphology.
Kumar et al. also used the co-precipitation method with magnesium nitrate as the precursor and ammonium hydroxide solution as the precipitating agent. This resulted in particles with an average size of about 11 nm.
Tandon and Chauhan also prepared MgO nanotubes using magnesium acetate and sodium hydroxide. They determined the average crystalline size to be 34.04 nm using XRD. From the FESEM results, tubular morphology is evident, with an outer diameter of approximately 78 nm and an inner diameter of 31 nm. The authors also reported a wider bandgap than the previous case (5.73 eV).
Sol-Gel Method
The sol-gel method is one of the most fundamental approaches aimed at forming new material structures (primarily metal oxides and similar inorganic materials) in the presence of an inorganic precursor and an organic solvent. The sol-gel approach was first introduced in the mid-19th century to produce silica gel. Metal alkoxides together with suitable solvents and reactants can form homogeneous solutions, which can then form colloidal suspensions (sol) and eventually polycondense into integrated networks (gel), which are then transformed into xerogels or aerogels depending on the drying method.
Mustuli et al., who focused on the production of nanostructured nanoparticles with the sol-gel method, also found that using magnesium acetate tetrahydrate together with a complexing agent in the form of oxalic acid and tertiary acid could inhibit crystal growth from achieving a thermally stable nanostructure with uniform nanoparticle size distribution.
Nanoparticles were synthesized from the same precursor and complex agent by Sutapa et al. These researchers additionally described stress, strain, and crystal energy and achieved the formation of cubic-shaped crystals, which they verified by SEM and documented the highest texture coefficient value (0.98 in the crystal plane (222)).
Solvo- and Hydrothermal Method
The solvothermal method is another of the most widely used methods for controlling the crystal growth of various materials. When a precursor and suitable solvent are placed in an autoclave under simultaneous exposure to higher temperature and pressure, the desired products are formed. These reaction conditions (temperature and pressure) allow the formation of high crystallinity materials compared to the co-precipitation method. In the case of the definition of the terminology of the “solvothermal” method, a medium other than water (e.g., alcohols or organic and inorganic solvents) is generally used as the solvent. In cases where water is used as the solvent, this method can be defined as “hydrothermal”.
Devaraja et al. described the properties of a nanocrystalline MgO powder prepared from magnesium nitrate hexahydrate and sodium hydroxide. Their presented procedure resulted in porous magnesium oxide particles with an average crystallite size of 25 nm. Among the other reported properties of the synthesized particles was the optical energy bandgap (5.5 eV).
References:
[1] HORNAK J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review.[J]. International Journal of Molecular Sciences, 2021, 22 23. DOI:10.3390/ijms222312752.You may like
Related articles And Qustion
See also
Lastest Price from Magnesium oxide manufacturers

US $10.00/kg2025-04-21
- CAS:
- 1309-48-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON

US $100.00-75.00/kg2025-04-21
- CAS:
- 1309-48-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000