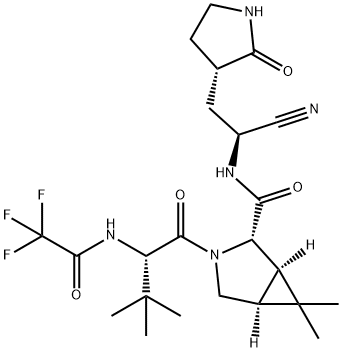
How to synthesise Nirmatrelvir on a large scale?
Synthesis of Nirmatrelvir
Nirmatrelvir is synthesised using saponification of bicycloproline ester. The specific synthesis steps are as follows:
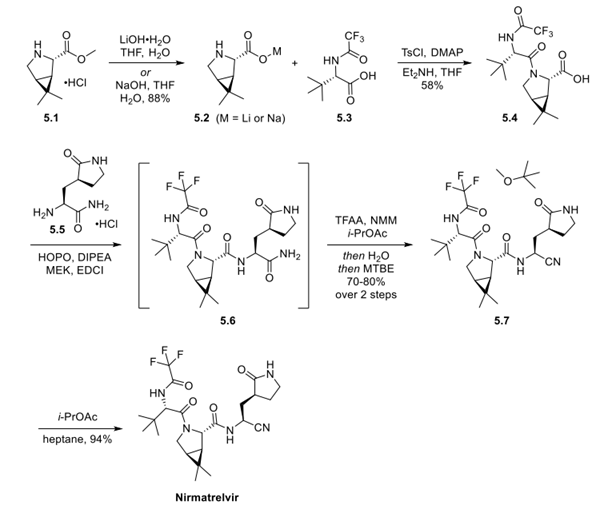
The synthesis was initiated by saponification of bicycloproline ester 5.1. Isolation of either the lithium orsodium salt of 5.2 was described, and the intermediate was isolated on scale. Next, acid 5.3 was treated with ptoluenesulfonyl chloride and DMAP, followed by the addition of amino acid 5.2 to give dipeptide 5.4 in 58% yield. Acid 5.4 was then treated with 2-hydroxypyridine-N-oxide and DIPEA in MEK, followed by the sequential addition of amine 5.5 and EDCI to give tripeptide 5.6, which was not isolated. Dehydration of the primary amide to the nitrile was accomplished by treating intermediate 5.6 with trifluoroacetic anhydride and NMM in isopropyl acetate. Solvent exchange with MTBE after quenching with water gave the nitrile 5.7 as the MTBE solvate in 70−80% yield for the 2 steps. Crystallization of 5.7 from isopropyl acetate in heptane produced nirmatrelvir in 94% yield.
Two Approaches to Bicyclic Pyrrolidine 5.1
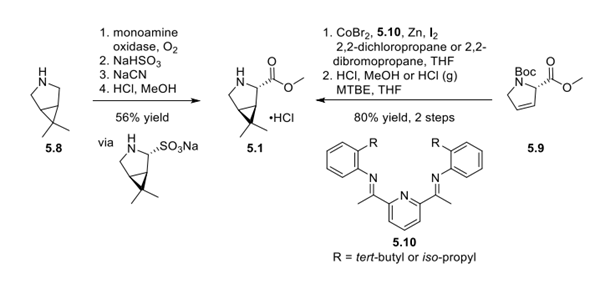
Building on this prior experience, chiral bicyclic pyrrolidine derivative 5.1 was manufactured via monoamine oxidase oxidation to desymmetrize 5.8, followed by cyanation and hydrolysis to the methyl ester found in 5.1. To diversify the supply chain for 5.1, an alternative route beginning with hydroxy-prolinederived olefin 5.9 was also developed and scaled to make metric ton quantities of pyrrolidine 5.1. Cobalt-catalyzed cyclopropanation using ligand 5.10 and 1,1-dichloropropane or 1,1-dibromopropane, followed by Boc deprotection, provided 5.1 in 80% yield over 2 steps.
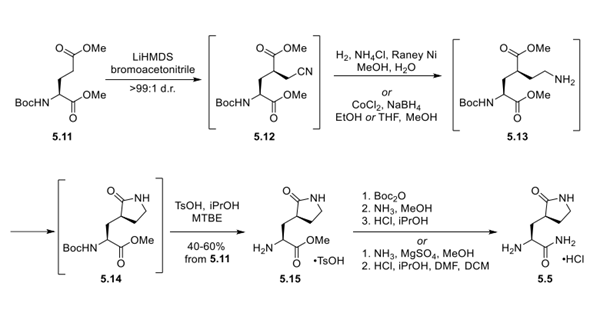
Synthetic Route to Aminolactam 5.5
First, careful control of temperature in the diastereoselective alkylation of glutamic acid derivative 5.11 using LiHMDS as base provided nitrile 5.12 in >99:1 d.r. Due to poor crystallinity, intermediate 5.12 was telescoped forward to a reduction using either hydrogenation with Raney nickel or cobalt and sodium borohydride to access amine 5.13, which could readily cyclize to lactam 5.14. Removal of the Boc protecting group using pTsOH enabled the isolation of the tosylate salt of 5.15 as a stable, crystalline solid. Finally, conversion of the methyl ester 5.15 to the primary amide 5.5 was reported to be conducted either by sequential Boc protection, aminolysis, and Boc deprotection or by direct aminolysis mediated by MgSO4. Yields were not yet available for the conversion of ester 5.15 to amide 5.5.
You may like
Related articles And Qustion

US $0.00/kg2025-07-06
- CAS:
- 2628280-40-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 2628280-40-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 2500kg