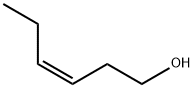
How to synthesis Leaf alcohol?
Introduction
Leaf alcohol is a flavour and fragrance compound. It has been shown to possess an intense characteristic grassy-green odour of freshly cut green grass and leaves. It is widely used as an added flavour in fragrances, food, and tobacco flavours to provide a fresh, grassy note[1].

Chemical property
Leaf Alcohol (Leaf Alcohol) [ scientific name: cis-3-hexanol (Cis-3-Hexenol); c6H12(ii) a The molecular weight is 100.16;]when the green grass is pure and high in concentration, the green grass has ether-like green leaf fragrance, and when the green grass is diluted, the green grass has a fresh and relaxed natural effect, such as the green grass is placed in the summer; d20: 0.846; bp: 156-157 ℃; flash point: 44 ℃, solubility: slightly soluble in water, soluble in ethanol, organic esters, ketones, alcohols and glycols[2].
Synthesis method
Hence, it is vital to research the synthesis of Leaf alcohol. At present, there are two processes used in industrial production: the process for the hydrogenation of 3-hexyn-1-ol and the process for the ring-opening reaction of 6-methyl-3,6-dihydro-2H-pyran. The shortage is due to certain difficulties and shortcomings in the current methods of synthesizing leaf alcohol. The disadvantages of the process for the hydrogenation of 3-hexyn-1-ol are the production security and dependability and the prime cost of catalyst and equipment. The disadvantages of the process for the ring opening reaction of 6-methyl-3,6-dihydro-2H-pyran are the product quality, production dependability, and the prime cost of the equipment. To solve this contradiction between supply and demand, many research groups are investigating chemical synthetic methods that could be applied in industrial production easily and economically.
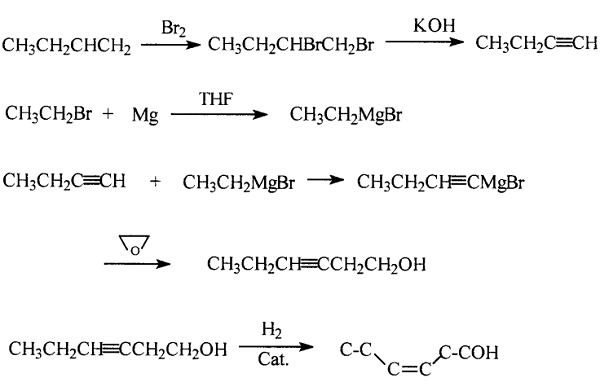
The process for the hydrogenation of 3-hexyn-1-ol is specifically to use butene-1 as raw material, brominate it, debrominate it under alkaline conditions to obtain butyne-1, then exchange butyne-1 and bromoalkane under the action of Grignard reagent, react with ethylene oxide, and hydrolyze to obtain 3-hexyne-1-ol; then incompletely hydrogenate it under normal temperature and pressure conditions to obtain cis-3-hexene-1-ol with a purity of more than 99%.
References:
[1] W. OU R W H Liu. A Review on Chemical Synthesis of Leaf Alcohol[J]. Current Organic Chemistry, 2022. DOI:10.2174/1385272827666221103102328.[2] T. KAJIWARA. Leaf Alcohol: Part XVIII. Condensation of Leaf Aldehyde by Means of Diethylamine[J]. Agricultural and biological chemistry, 1969, 11 1: 221-226. DOI:10.1080/00021369.1969.10859322.
You may like
Related articles And Qustion
See also
Lastest Price from Leaf alcohol manufacturers

US $0.00/KG2025-04-21
- CAS:
- 928-96-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000kg/month

US $0.00/Kg/Drum2025-04-21
- CAS:
- 928-96-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200mt/year