How is Sotorasib synthesised?
Synthesis of Sotorasib
The synthetic route of Sotorasib is completed in three steps, starting with the amidation of nicotinic acid derivatives to produce nicotinamide, obtaining acyl isomer purity 28.6 by classical resolution, followed by the preparation of Boroxine Reagent, and finally preparing it by coupling, acid treatment and other reactions. The specific synthesis steps are as follows:
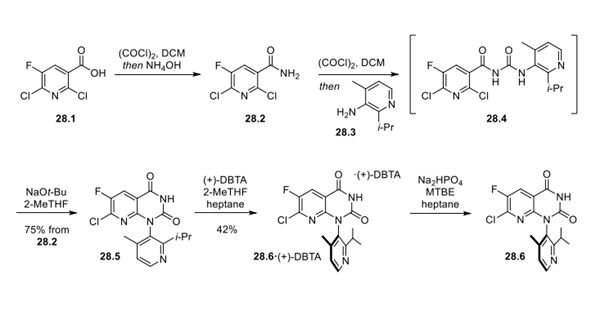
Step 1: Accessing Atropisomerically Pure 28.6 via Classical Resolution
The synthetic route began with amidation of nicotinic acid derivative 28.1 to form nicotinamide 28.2. Treatment with oxalyl chloride followed by addition of aminopyridine 28.3 provided urea 28.4 via an isocyanate intermediate. Urea 28.4 was not isolated and instead was telescoped into a base-mediated cyclization to form pyrimidine dione 28.5. At this point, classical resolution by treatment with (+)-2,3-dibenzoyl-D-tartaric acid ((+)-DBTA) in 2-MeTHF and heptane allowed for isolation of the desired atropisomeric co-crystal 28.6 (2-MeTHF solvate) in >2000:1 ratio, which could be isolated as the free base by pH adjustment with disodium monophosphate and isolation from MTBE and heptane. This resolution has been demonstrated on >500 kg scale. To improve the throughput of the process, the undesired atropisomer could be recycled from the mother liquors of the filtration of 28.6 by first performing a salt break to remove excess (+)-DBTA and then subjecting the free base to high temperature (300 °C) in anisole to provide racemic 28.5.
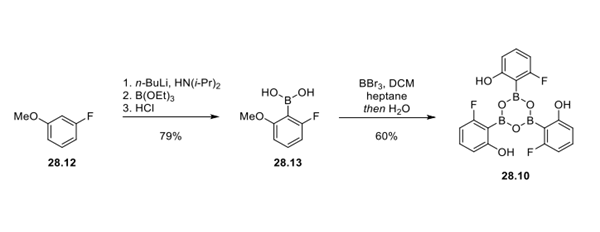
Step 2: Access to Boroxine Reagent 28.10
A gram-scale synthesis of boroxine 28.10 is outlined. 3-Fluoroanisole 28.12 was deprotonated with LDA at cryogenic temperatures and quenched with triethylborate, which provided boronic acid 28.13 after treatment with HCl. Demethylation with boron tribromide provided boroxine 28.10 in 60% yield.
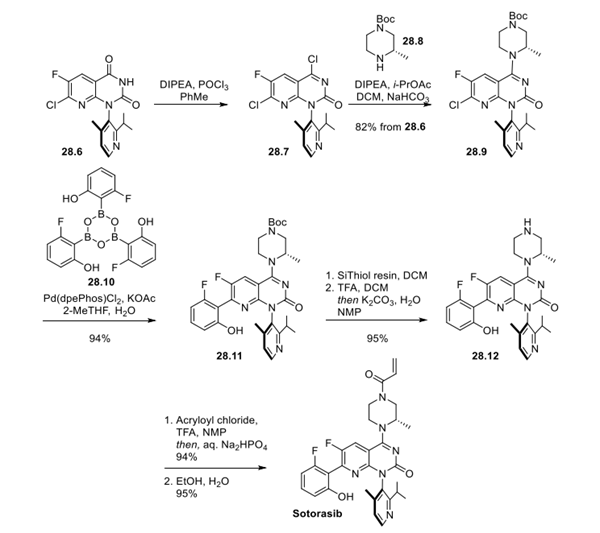
Step 3:Preparation of Sotorasib
Treatment of dione 28.6 with POCl3 provided chloride 28.7, which set the stage for the subsequent SNAr reaction with piperazine derivative 28.8 to furnish pipazoline 28.9. Suzuki coupling with boroxine 28.10 formed intermediate 28.11. Next, dissolution of 28.11 and treatment with SiThiol scavaenger removed residual Pd, and addition of TFA cleaved the Boc protecting group and provided amine 28.12, which underwent amidation using acrolyl chloride to form the acrylamide found in Sotorasib. A seeded recrystallization from ethanol and water provided the final product, sotorasib, in a 65% overall yield from intermediate 28.6.