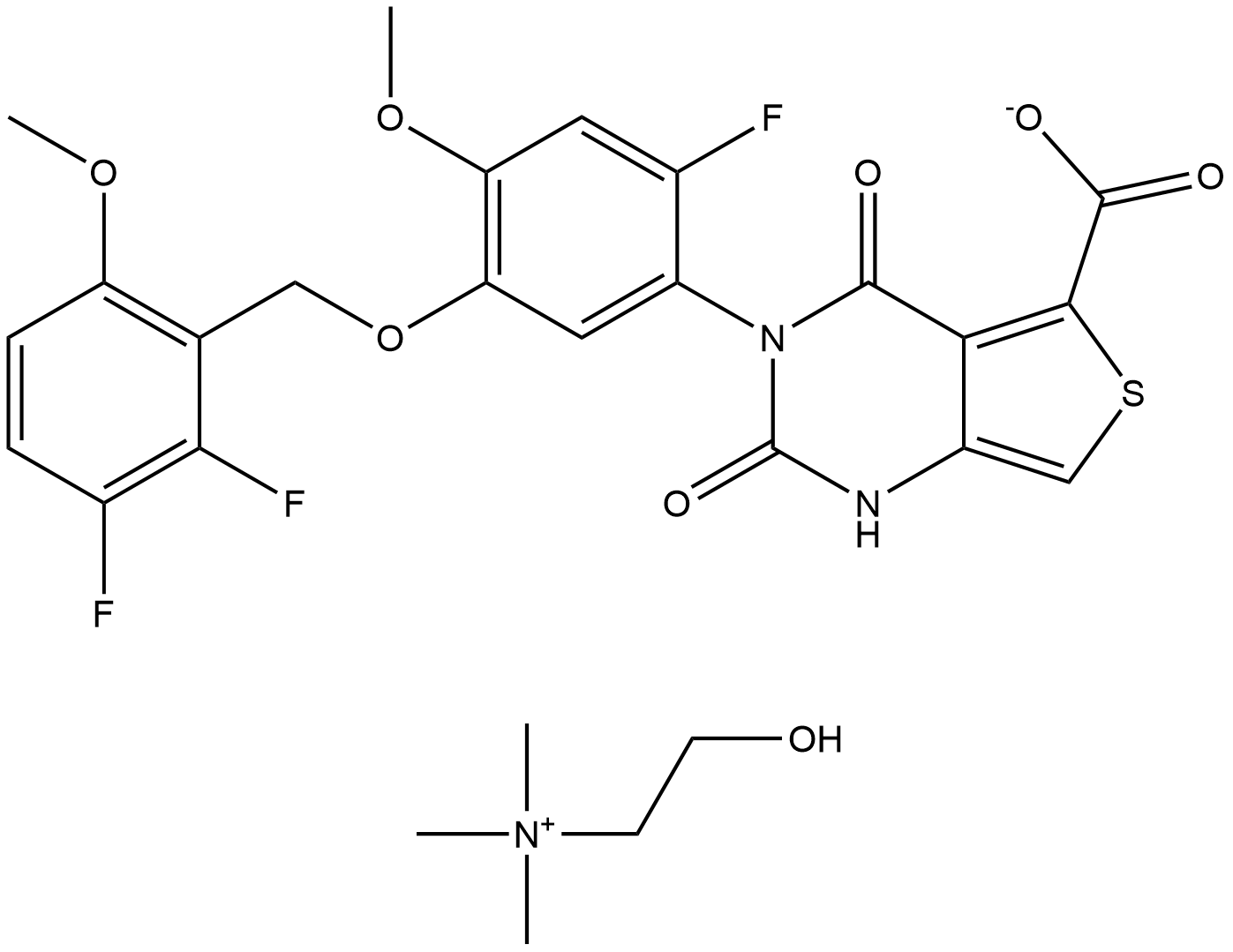
How is Linzagolix choline synthesised?
Synthesis of Linzagolix choline
The synthesis of Linzagolix choline was carried out by an efficient two-component polymerisation synthesis. The specific synthesis steps are as follows:
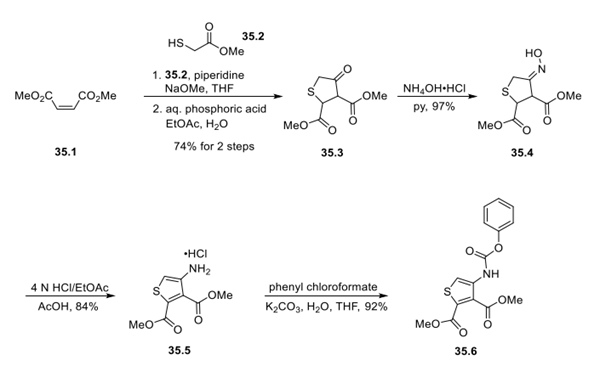
Step 1: Synthesis of Intermediate 35.6
Synthesis of the first component, intermediate 35.6, began with the reaction of dimethyl maleate 35.1 and methylthioglycolate 35.2 under basic conditions. Upon exposure to mildly acidic conditions, cyclic mercaptan 35.3 was afforded as a crystalline solid. An aromatization was then conducted to convert this cyclic thiol to the corresponding thiophene. Conversion of the ketone 35.3 to the corresponding oxime by treatment with hydroxylamine hydrochloride in warm pyridine, followed by subjection of the resulting oxime to strongly acidic conditions, furnished aminothiophene 35.5, a sequence referred to as a Semmler−Wolff aromatization. Lastly, 35.5 was exposed to phenyl chloroformate to give carbamate intermediate 35.6 in 92% yield.
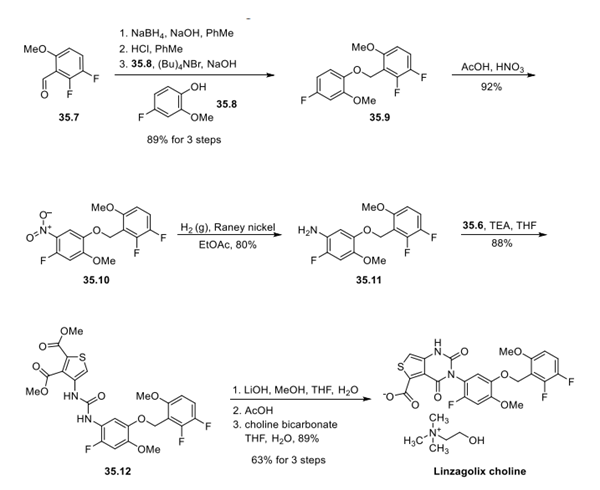
Step 2: Synthesis of Intermediate 35.11 and Linzagolix Choline
The synthesis of the second component of the convergent approach, intermediate 35.11, began with borohydride reduction of aldehyde 35.7, followed by chlorination of the resulting alcohol with concentrated HCl. The bromide was then generated in situ with the addition of tetrabutylammonium bromide, and ether formation was accomplished by addition of phenol 35.8 under basic conditions to give ether 35.9 in 89% yield over 3 steps. Standard nitration conditions were employed to give 39.10. Reduction to the corresponding amine is accomplished using Raney-Ni to give the key aniline intermediate 35.11. Convergence of the two advanced intermediates 35.6 and 35.11 proceeded at this stage under basic conditions to form the penultimate thiophenyl urea 35.12 in good yield. Exposure of this urea to lithium hydroxide followed by acidic conditions facilitated an intramolecular cyclization, after which treatment with choline bicarbonate allowed for precipitation of Yselty, which was isolated as the choline salt.