Pharmacology research of Nafamostat mesylate
Introduction
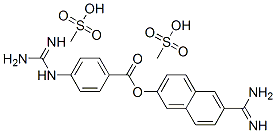
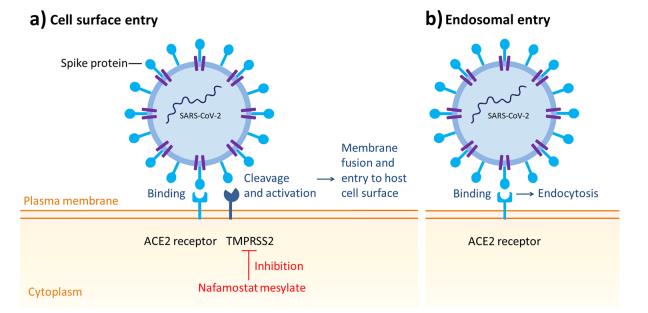
Nafamostat mesylate ((6-carbamimidoylnaphthalen-2-yl)4-(diaminomethylideneamino) benzoate) (Fig. 1) is a broad-spectrum synthetic serine protease inhibitor that has been widely used in Japan and Korea for the treatment of acute pancreatitis and disseminated intravascular coagulation. The dosing for nafamostat varies based on the indication. For acute pancreatitis, the approved mode of administration is 10 mg by intermittent intravenous (IV) infusion over 2h, once or twice daily according to the patient’s status. For disseminated intravascular coagulation,a continuous infusion of 0.06–0.20 mg/kg/h is used. Nafamostat is also approved as an anticoagulant therapy for patients with bleeding tendencies undergoing continuous renal replacement therapy or cardiopulmonary bypass.It has actions as an anticoagulant (it inhibits the activity of a variety of serine proteases generated during the coagulation cascade and the infammatory process, such as the activated factors VIIa and XIIa, kallikrein, thrombin, components of the complement system and trypsin), antifbrinolytic (it inhibits tissue-type and urokinase plasminogen activators) and antiplatelet. The indicated dose of nafamostat when used to prevent blood coagulation during extracorporeal blood circulation is from 20 to 50 mg/h continuous lyinfused.Nafamostat has been identifed as a potent inhibitor of the S-mediated membrane fusion, and manifests antiviral activity through inhibition of TMPRSS2 (Fig. 2).[1]

Nafamostat mesylate, an apparent soi-disant panacea of sorts, is widely used to anticoagulate patients undergoing hemodialysis or cardiopulmonary bypass, mitigate the inflammatory response in patients diagnosed with acute pancreatitis, and reverse the coagulopathy of patients experiencing the commonly preterminal disseminated intravascular coagulation in the Far East. The serine protease inhibitor nafamostat mesylate exhibits significant neuroprotective effects in the setting of neurovascular ischemia. Nafamostat mesylate generates neuroprotective effects by attenuating the enzymatic activity of serine proteases, neuroinflammatory signaling cascades, and the endoplasmic reticulum stress responses, downregulating excitotoxic transient receptor membrane channel subfamily 7 cationic currents, modulating the activity of intracellular signal transduction pathways, and supporting neuronal survival (brain-derived neurotrophic factor/TrkB/ERK1/2/CREB, nuclear factor kappa B.The effects collectively reduce neuronal necrosis and apoptosis and prevent ischemia mediated disruption of blood-brain barrier microarchitecture. Investigational clinical applications of these compounds may mitigate ischemic reperfusion injury in patients undergoing cardiac, hepatic, renal, or intestinal transplant, preventing allograft rejection, and treating solid organ malignancies. Neuroprotective effects mediated by nafamostat mesylate support the wise conduct of randomized prospective controlled trials in Western countries to evaluate the clinical utility of this compound.[2]
Nafamostat Mesylate for Treatment of COVID-19
Nafamostat mesylate (nafamostat) is a broad-spectrumsynthetic serine protease inhibitor that has been widely usedin Japan and Korea for non-infection indications such as pancreatitis and disseminated intravascular coagulation. It has demonstrated nanomolar potency in in vitro SARS-CoV-2 studies. In studies combining antiviral potency with achievable plasma concentrations, nafamostat is consistently one of the most potent drugs against SARS-CoV-2. However, its limited global licensing as well as its pharmacokinetic characteristics mean that it has not been extensively studied to date as a potential COVID-19 antiviral treatment.
Pharmacokinetics and Pharmacodynamics
Nafamostat is rapidly metabolised by esterases in circulating blood into 6-amidino-2-naphthol (AN) and 4-guanidinobenzoic acid (4-GBA), which are inactive proteaseinhibitors. Hyperkalaemia is a known side effect of nafamostat mesylate administration. Renal andextrarenal K+ imbalance have been described as mechanisms of hyperkalaemia related to nafamostat. Muto et al. reported that the two metabolites of nafamostat act on the apical membrane of the collecting duct cell and inhibit the amiloride-sensitive Na+ conductance, causing inhibition of K+ secretion. In addition, Ookawara et al. Reported that nafamostat and its metabolite AN inhibit erythrocyte potassium infux by suppressing the Na-K ATPase-dependent pathway.
In vitro human lung cell line studies with nafamostat demonstrate high antiviral potency against SARS-CoV-2 (half maximal inhibitory concentration [IC50] of 0.0022 µM [compared to remdesivir1.3 µM]), ostensibly via inhibition of the cellular enzyme transmembrane protease serine 2 (TMPRSS2) preventing viral entry into human cells. In addition, the established antithrombotic activity is hypothesised to be advantageous given thrombosis-associated sequelae of COVID-19. Clinical reports to date are limited, but indicate a potential benefit of nafamostat in patients with moderate to severe COVID-19.[1]
Nafamostat mesylate inhibits thrombin-mediated blood-spinal cord barrier breakdown
Time to first dose of nafamostat administration was tested on rats after contusive spinal cord injury (SCI). The optimal time window of nafamostat mesylate was screened by evaluating hindlimb locomotion and electrophysiology. As nafamostat mesylate is a serine protease inhibitor known to target thrombin, researchers used argatroban (Arg), a thrombin-specific inhibitor, as a positive control in the time window experiments. Western blot and immunofluorescence of thrombin expression level and its enzymatic activity were assayed at different time points, as well its receptor, the protease activated receptor 1 (PAR1) and downstream protein matrix metalloproteinase-9 (MMP9). Blood-spinal cord barrier (BSCB) permeability leakage indicator Evans Blue and fibrinogen were analyzed along these time points. The infiltration of peripheral inflammatory cell was observed by immunofluorescence. Results: The optimal administration time window of nafamostat mesylate was 2-12 h post-injury. Argatroban, the thrombin-specific inhibitor, had a similar pattern. Thrombin expression peaked at 12 h and returned to normal level at 7 days post-SCI. PAR1, the thrombin receptor, and MMP9 were significantly upregulated after SCI. The most significant increase of thrombin expression was detected in vascular endothelial cells (ECs). Nafamostat and argatroban significantly downregulated thrombin and MMP9 expression as well as thrombin activity in the spinal cord. Nafamostat inhibited thrombin enrichment in endothelial cells. Nafamostat administration at 2-12 h after SCI inhibited the leakage of Evans Blue in the epicenter and upregulated tight junction proteins (TJPs) expression. Nafamostat mesylate administration 8 h post-SCI effectively inhibited the infiltration of peripheral macrophages and neutrophils to the injury site. Conclusions: Our study provides preclinical information of nafamostat about the administration time window of 2-12 h post-injury in contusive SCI. Zhao et al. revealed that nafamostat functions through inhibiting the thrombin-mediated BSCB breakdown and subsequent peripheral immune cells infiltration.[3]
Nafamostat mesylate prevents metastasis and dissemination of neuroblastoma
Neuroblastoma is a highly malignant disease with a poor prognosis and few treatment options. Despite conventional chemotherapy for neuroblastoma, resistance, invasiveness, and metastatic mobility limit the treatment efficacy. Therefore, it is necessary to develop new strategies for treating neuroblastoma. The present study aimed to evaluate the anticancer effects of nafamostat mesylate, a previously known serine protease inhibitor, on neuroblastoma cells. Effects of nafamostat mesylate on neuroblastoma cell migration and proliferation were analyzed by wound healing assay and WST-8 assay, respectively. To elucidate the mechanisms underlying the effects of nafamostat mesylate on neuroblastoma, the expression levels of NF-κB were measured via western blotting, and the production of the cytokine vascular endothelial growth factor (VEGF) in the cell culture supernatants was determined via ELISA. In addition, a mouse model of hematogenous metastasis was used to investigate the effects of nafamostat mesylate on neuroblastoma. It was determined that nafamostat mesylate significantly inhibited migration and invasion of Neuro-2a cells, but it had no effect on cell proliferation at 24 h after treatment. Exposure of Neuro-2a cells to nafamostat mesylate resulted in decreased vascular endothelial growth factor production, which could be a pivotal mechanism underlying the inhibitory effects of neuroblastoma metastasis. The results of the present study suggest that nafamostat mesylate may be an effective treatment against neuroblastoma invasion and metastasis.[4]
References
1. Hernández-Mitre MP, Tong SYC, Denholm JT, et al. Nafamostat Mesylate for Treatment of COVID-19 in Hospitalised Patients: A Structured, Narrative Review. Clin Pharmacokinet. 2022;61(10):1331-1343. doi:10.1007/s40262-022-01170-x
2. Ghali GZ, Ghali MGZ. Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia [retracted in: Neural Regen Res. 2022 May;17(5):958. doi: 10.4103/1673-5374.295353.]. Neural Regen Res. 2020;15(12):2217-2234. doi:10.4103/1673-5374.284981[Retracted]
3. Zhao C, Zhou T, Zhao X, et al. Delayed administration of nafamostat mesylate inhibits thrombin-mediated blood-spinal cord barrier breakdown during acute spinal cord injury in rats. J Neuroinflammation. 2022;19(1):189. Published 2022 Jul 16. doi:10.1186/s12974-022-02531-w
4. Morimoto M, Toyoda H, Niwa K, et al. Nafamostat mesylate prevents metastasis and dissemination of neuroblastoma through vascular endothelial growth factor inhibition. Mol Clin Oncol. 2022;17(3):138. Published 2022 Jul 21. doi:10.3892/mco.2022.2571
You may like
See also
Lastest Price from Nafamostat mesylate manufacturers

US $5.00-0.50/KG2025-06-11
- CAS:
- 82956-11-4
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $0.00-0.00/g2025-04-21
- CAS:
- 82956-11-4
- Min. Order:
- 1g
- Purity:
- 98.5~101.5%
- Supply Ability:
- 10kg/month