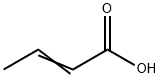
How is Crotonic acid produced?
Crotonic acid is mainly used as a comonomer with vinyl acetate. The resulting copolymers are used in paints and adhesives. Crotonyl chloride reacts with N-ethyl-2-methylaniline (N-ethyl-o-toluidine) to provide crotamiton, which is used as an agent against scabies.
The preparation method of crotonic acid
After distilling off unreacted crotonaldehyde, the reaction mixture obtained by oxidizing crotonaldehyde with air or oxygen contains more than 90% by weight of trans-crotonic acid. The rest is mainly cis-crotonic acid, with small amounts of crotonaldehyde, formic acid, acetic acid and propionic acid.
To obtain pure trans-crotonic acid from this mixture, for example for use as a monomer in polymers or as a builder unit in synthesis, the mixture water is worked up in a two-stage purification operation involving fractional distillation and crystallization.
However, during crystallization from water, approximately one ton of trans-crotonic acid forms approximately one ton of highly contaminated effluent, which must be biologically purified. Each ton of dry and wet trans-crotonic acid produces about 1,500 cubic meters (S.T.P.) of air, which must also be purified. Furthermore, crystallization from water also leads to product loss, and finally, the drying of water-wet crotonic acid requires a lot of energy.
Surprisingly, it has now been found that these disadvantages can be avoided if the crystallization is performed from a melt rather than from water. In this way, not only trans-crotonic acid can be separated, but also high-purity cis-crotonic acid can be separated under mild conditions.
Incompatibilities of crotonic acid
Crotonic acid may possibly form explosive mixtures with air. It is a strong reducing agent and its aqueous solution is a weak acid. It reacts violently with oxidants, combustibles, and strong bases. Moisture or intense sunlight (UV) may cause explosive polymerization. May accumulate a static charge and its vapors may ignite. Combustible when exposed to heat or open flame. Carboxyl compounds react with all bases, both inorganic and organic (i.e. amines), releasing large amounts of heat, water and potentially harmful salts. Incompatible with arsenic compounds 938 Crotonic acid (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, sulfides (releases heat, toxic gases and possibly flammable gas), thiosulfate and dithionite (releasing bisulfate and sulfur oxides) ).
You may like
Related articles And Qustion
Lastest Price from Crotonic acid manufacturers

US $10.00/kg2025-04-21
- CAS:
- 3724-65-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 3724-65-0
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons