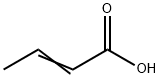
Crotonic acid: Origin, Aplications in Western Blotting and Biosynthesis
General Description
Crotonic acid has a dual origin rooted in both the natural world and chemical synthesis. It was initially identified as a plant growth inhibitory factor in carrot seeds and found in plants like Croton tiglium. Its versatility is evident in its use in organic synthesis for pharmaceuticals, fragrances, and polymers. In western blotting, crotonic acid has been developed as a method to inhibit horseradish peroxidase (HRP) in sequential detections without losing transferred proteins. Furthermore, metabolic engineering of Yarrowia lipolytica has enabled environmentally friendly and large-scale industrial production of crotonic acid, with significant yield improvements through genetic modifications.

Figure 1. Crotonic acid
Origin
Crotonic acid, also known as (E)-2-butenoic acid, has its origin rooted in the natural world as well as in chemical synthesis. This compound was first identified as the plant growth inhibitory factor in water extracts from Perfekcja carrot seeds, belonging to the Daucus carota L. species. However, it is not exclusive to Perfekcja carrots and can also be found in other late varieties of carrots. The herbicidal properties of crotonic acid, particularly its role as an allelopathic and autotoxic factor within seeds, hinder the growth of various plant species including cress, cucumber, onion, and carrot. Its presence in seed extracts points to its significant role in mediating allelopathic interactions of carrot seeds with other plants. On the other hand, crotonic acid was discovered by French chemist Auguste Laurent in 1840 during his study of crotonaldehyde. It can be naturally found in plants like Croton tiglium and is widely used in organic synthesis for pharmaceuticals, fragrances, and polymers due to its reactivity and unique chemical properties. Crotonic acid can be derived from crotonaldehyde through oxidation processes or synthesized industrially using chemical reactions involving acetic acid and acetylene. This dual origin, both natural and synthetic, underscores the versatility and importance of crotonic acid in both biological and industrial contexts. 1
Applications in western blotting
Crotonic acid has been successfully developed as an alternative to stripping for inhibiting horseradish peroxidase (HRP) in sequential detections of chemiluminescent western blotting (WB). Inactivation of HRP is a conventional method used to strip western blot membranes for reprobing different proteins. However, little evidence exists on whether other chemical substances can affect the biological activity of HRP during stripping and re-probing of WB blots. The use of 20% Crotonic acid inhibits HRP used in sequential chemiluminescent WB on polyvinylidene difluoride (PVDF) and Nitrocellulose (NC) membrane without losing transferred proteins and damaging the membrane. Moreover, NC blots incubated in Crotonic acid (40 °C, 30min) allows for three rounds of HRP inhibition in sequential detections, thus saving time and valuable samples without the need to rerun gels. This method is a significant breakthrough in the field of western blotting, providing a more efficient and convenient solution for re-probing WB blots, especially for researchers studying protein-protein interactions in different fields such as biochemistry and molecular biology. 2
Biosynthesis
The biosynthesis of crotonic acid was achieved through metabolic engineering of Yarrowia lipolytica using the butanol-forming route. In this study, genes encoding crotonase and 3-hydroxybutyryl-CoA dehydrogenase from Clostridium beijerinckii, as well as the thioesterase gene from Bacteroides thetaiotaomicron, were heterologously expressed in Y. lipolytica. The engineered strain LZJ001 successfully accumulated 62.3 ± 4.2 mg/L of crotonic acid. Subsequently, overexpression of acetyl-CoA acetyltransferase from Saccharomyces cerevisiae in strain LZJ002 led to an increased production of 123.5 ± 6.8 mg/L of crotonic acid. Further expression of pyruvate dehydrogenase from Escherichia coli in the fully engineered strain LZJ004 resulted in a significant boost, producing 220.0 ± 8.2 mg/L of crotonic acid in shaking-flask culture, representing a 3.5-fold increase over the initial strain. This approach sets the stage for environmentally friendly and large-scale industrial production of crotonic acid. 3
Reference
1. Jasicka-Misiak I, Wieczorek PP, Kafarski P. Crotonic acid as a bioactive factor in carrot seeds (Daucus carota L.). Phytochemistry. 2005;66(12):1485-1491.
2. Zhang C, Li Q, Zhan L, Wang XL, Zhang JJ, Xu CJ. Inactivation of horseradish peroxidase with crotonic acid for reprobing of western blotting. Anal Biochem. 2023;665:115070.
3. Wang L, Zong Z, Liu Y, et al. Metabolic engineering of Yarrowia lipolytica for the biosynthesis of crotonic acid. Bioresour Technol. 2019;287:121484.
Related articles And Qustion
See also
Lastest Price from Crotonic acid manufacturers

US $10.00/kg2025-04-21
- CAS:
- 3724-65-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 3724-65-0
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons