Hafnium Tetrachloride: An Essential Compound in Modern Chemistry
Introduction
Hafnium tetrachloride (HfCl4) is a metal inorganic salt compound commonly used as a precursor for the production of hafnium metal and various hafnium compounds. In addition, it is a catalyst for certain organic synthesis reactions.

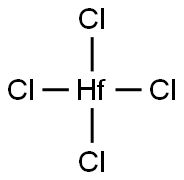
Figure 1 Characteristics of Hafnium tetrachloride
Properties of Hafnium Tetrachloride
Hafnium tetrachloride has the appearance of a white crystalline solid or powder with a melting point of 434 °C. In chemical vapour deposition (CVD), the sublimation of HfCl4 powder occurs mainly in the temperature range of 200-300 °C. HfCl4 is used as a precursor for HfS2 and is preferred on sapphire (Al2O3) substrates to enhance the crystallinity of the films. In the chemical vapour deposition (CVD) of HfS2, HfCl4 is usually used as the Hf precursor and a sapphire (Al2O3) substrate is preferred in order to improve the crystallinity of the films.
Possible reactions
The formation of hafnium dioxide in the reaction of
hafnium tetrachloride with water is described by the following net reaction:
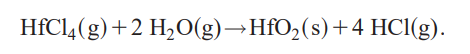
The (g) refers to gas phase and the (s) to solid phase. In
ALD, this net reaction is divided into two successive half-reactions, the first half-reaction being the reaction of HfCl4
and the second half-reaction being the reaction of
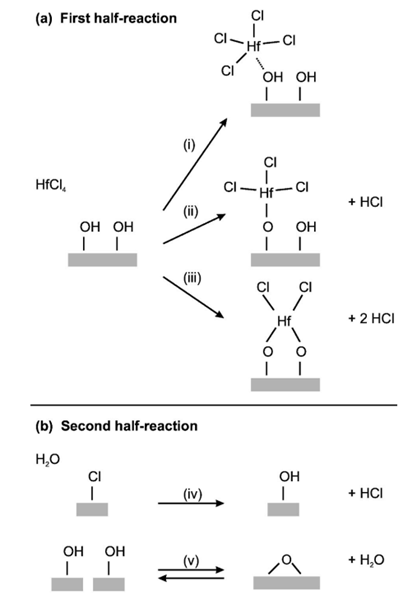
H2O. Some reactions that typically occur during ALD, and which can occur during the two half-reactions of the HfCl4 /H2O process, are shown in Fig. 2. In
the first half-reaction, HfCl4 may associate on hydroxyl(OH)
groups or other oxygen sites [reaction i in Fig. 2], and
react through ligand exchange with one [reaction ii] or two [reaction iii] surface OH groups. In the second
half-reaction, H2O replaces the surface chlorine(Cl) groups
with OH groups [reaction iv]. Two neighboring OH
groups may react to release water [reaction v], or, in a
reverse reaction, water can react dissociatively with an oxygen bridge, forming two OH groups.
Applications of Hafnium Tetrachloride
Hafnium tetrachloride is used as an organic synthesis intermediate for the preparation of other heterocyclic compounds.HfCl4 reacts with tris(trimethylsilyl)silyl potassium tmen adduct to form the [tris(trimethylsilyl)silyl] trichlorohafnium tmen complex, which also reacts with 2,6-dimethylphenylisocyanide leading to the insertion of a hafnium-silicon bond.
α-Aminonitrile was synthesised by a three-component Strecker reaction using hafnium tetrachloride as a catalyst at room temperature using acetaldehyde or cyclic acetaldehyde, curious aromatic amines and trimethylsilyl cyanide (TMSCN). In addition, it was also used in the study of methods for the partial separation of hafnium from zirconium.
References:
[1] A. A. PALKO D W K A D Ryon. The Vapor Pressures of Zirconium Tetrachloride and Hafnium Tetrachloride[J]. The Journal of Physical Chemistry , 1958, 62 3: 257-384. DOI:10.1021/j150561a017.[2] A. A. PALKO D W K A D Ryon. The Vapor Pressures of Zirconium Tetrachloride and Hafnium Tetrachloride[J]. The Journal of Physical Chemistry , 1958, 62 3: 257-384. DOI:10.1021/j150561a017.
[3] PUURUNEN R. Analysis of hydroxyl group controlled atomic layer deposition of hafnium dioxide from hafnium tetrachloride and water[J]. Journal of Applied Physics, 2004, 95 1: 4777-4786. DOI:10.1063/1.1689732.
[4] PUURUNEN R. Analysis of hydroxyl group controlled atomic layer deposition of hafnium dioxide from hafnium tetrachloride and water[J]. Journal of Applied Physics, 2004, 95 1: 4777-4786. DOI:10.1063/1.1689732.
[5] FRANK D, BAUMGARTNER J, MARSCHNER C. First successful reaction of a silyl anion with hafnium tetrachloride[J]. Chemical Communications, 2002, 11: Page 1159 to 1241. DOI:10.1039/B201508K.
[6] XUELIN ZHANG Q Z Qin pei Wu. Efficient Three-Component Strecker Reaction of Acetals and Aromatic Amines Catalysed by Hafnium Tetrachloride at Room Temperature[J]. Journal of Chemical Research-s, 2013, 54 1: 1576-1580. DOI:10.3184/174751913X13814077309487.
Related articles And Qustion
See also
Lastest Price from Hafnium(IV) chloride manufacturers

US $5.00/KG2025-03-28
- CAS:
- 13499-05-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS

US $0.00/Kg/Drum2025-02-19
- CAS:
- 13499-05-3
- Min. Order:
- 10Kg/Drum
- Purity:
- 99.5%
- Supply Ability:
- 300tons