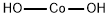High Purity Cobalt Hydroxide: A Detailed Overview
Cobalt hydroxide is an important chemical raw material and has been used to manufacture rechargeable batteries such as MH-Ni and lithium-ion batteries. Cobalt hydroxide can also be used as a desiccant, catalyst or catalyst product precursor for paint films and varnishes, and is used to produce other cobalt compounds.

Figure 1 Characteristics of high-purity Cobalt hydroxide
There are two difficulties in the production of cobalt hydroxide:
(1) Cobalt hydroxide is easy to form colloids or flocs when precipitated, which is not easy to filter;
(2) Cobalt hydroxide does not react completely under low alkaline conditions and has a high impurity content; it is easily oxidized to brown-black Co(OH)3 by oxygen in the air in high alkaline solutions. At present, the preparation methods of Co(OH)2 include alkali precipitation method, electrolytic synthesis method, precipitation conversion method, etc.
Traditional preparation method
Alkaline precipitation method can synthesize cobalt hydroxide from cobalt salt solution and alkali solution. When the alkalinity is low, the reaction is incomplete and the product contains alkaline salts, resulting in an excessively high anion content; when the alkalinity is high, although it is conducive to the conversion of alkaline salts into cobalt hydroxide, cobalt hydroxide is very sensitive to oxidation under alkaline medium conditions, so it is easily oxidized during aging, filtration, washing, etc. Xu Qiuhong et al. published a paper entitled "Research on New Process for Synthesis of β-Co(OH)2" in Applied Chemical Industry (2006, 35 (7): P504-506). Although ammonia will volatilize and take away some air during the preparation process, the divalent cobalt ammonia complex ion is unstable and easily oxidized to a trivalent state.
OMG Finland has published patent application CN1359353, entitled "Preparation method of high-density large-particle cobalt hydroxide or cobalt mixed hydroxide and products prepared by this method", which does not involve the anti-oxidation problem of cobalt hydroxide.
H.C.Stark's patent application CN1190947 discloses the use of precipitation conversion method to prepare cobalt hydroxide. This method has a long process flow and harsh drying conditions, which is not conducive to industrial production.
New preparation method
The technical problem to be solved by the present invention is to overcome the defects of the above-mentioned prior art and provide a method for preparing dense single crystal cobalt hydroxide and preventing oxidation. The process conditions are easy to control and suitable for large-scale industrial production.
To this end, the present invention adopts the following technical scheme: a method for preparing dense crystalline cobalt hydroxide, characterized in that: a cobalt salt solution is used as a base liquid, a weak alkaline substance is added to the base liquid under stirring conditions, a precipitation reaction occurs, and a basic salt turbid liquid α-Co(OH)2 is generated; then NaOH solution and a protective agent solution are continuously injected into the turbid liquid to replace the anions in the cobalt basic salt, and the pH value is adjusted to convert α-Co(OH)2 into pink β-Co(OH)2, and the obtained cobalt hydroxide is a dense single crystal particle.
Lastest Price from Cobalt(II) hydroxide manufacturers

US $0.00-0.00/KG2025-12-05
- CAS:
- 21041-93-0
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $79.00-38.00/kg2025-04-21
- CAS:
- 21041-93-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton