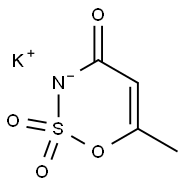
Guardians of Sweet Secrets: Acesulfame Potassium
Abstract
In the vast universe of food science, there is a magical substance that has become an indispensable "sweet guardian" in the modern food industry with its extraordinary sweetness, excellent stability, and a wide range of applications -- Acesulfame Potassium, Chinese name potassium acetylsulfanilate, more affectionately known as "Acesulfame". Today, let's uncover the mystery of this "sweet guardian" and explore the scientific mysteries and wide applications behind it.
Birth and Naming
The story of Acesulfame Potassium began in 1967 in a Hearst laboratory in Germany. In an accidental experiment, scientists created an unprecedented compound-potassium acetylsulfanilate - through the ingenious combination of fluorosulfonyl isocyanate or chlorosulfonyl isocyanate with various active methylene compounds. This discovery quickly attracted the attention of the food industry, because it not only has a far greater sweetness than sucrose but also has extremely high stability and safety.
In naming, the scientific and precise word Acesulfame Potassium (Ace-K for short) not only reflects the characteristics of its chemical structure but also contains scientists' expectations for its future application. The Chinese name "Ansai Mi" is more poetic and friendly, meaning that it can bring safe, pure, and lasting sweet enjoyment of food.1
Chemical properties
The molecular formula of Acesulfame Potassium is C4H4KNO4S with a relative molecular mass of 201.24. It is a colorless or white odorless crystalline powder. One of its most striking properties is its astonishing sweetness-about 200 times that of sucrose, meaning that very small amounts added to food can achieve the desired sweetness effect. In addition, it also has good solubility, easy to dissolve in water, and difficult to dissolve in organic solvents such as ethanol, which makes its application in food processing more flexible.
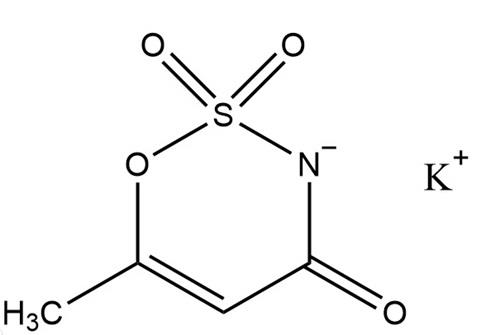
It also excels in terms of stability. It is light and heat resistant and can keep its sweet taste unchanged even under high temperatures or long periods of light. This high stability makes it an ideal sweetener for baked goods, beverages, and other products that need to be processed at high temperatures.2
Security assessment
Since the discovery of Acesulfame Potassium, its safety has been the focus of scientific and public concern. After years of in-depth research and extensive practice, existing research shows that it does not cause harm to human health when used reasonably in accordance with standard provisions. Food safety regulators in various countries have also approved its use as a food additive, such as the United Kingdom in 1983 and China in 1992.
GB2760-2014 "Food Safety National Standard for the use of food additives" clearly specifies its scope of use and maximum amount of addition, to ensure that its application in a variety of food is safe and reasonable. At the same time, the standard also stipulates that the daily allowable intake of it is 0 to 15 mg/kg of body weight, providing scientific health guidance for consumers.3
Wide application
Due to its outstanding sweetness, stability, and safety, Acesulfame Potassium is widely used in the food industry. From beverages and sweets to pastries and condiments, it can be found in almost any food that needs to be sweetened.
In the beverage sector, it is favored for its high sweetness and low-calorie properties. Whether it is carbonated drinks, fruit drinks, or energy drinks, adding it can reduce sugar intake without affecting the taste and meet the needs of modern consumers for a healthy diet.
In the field of confectionery and pastry, it is known for its stability and high-temperature resistance. During the candy-making process, it is able to keep its sweetness unaffected by high temperatures; In the process of pastry baking, it can also stably play its sweet role, making the pastry taste more delicate and sweet.
In addition, it is also widely used in condiments, table sweeteners, and some processed foods. Such as soy sauce, jam, preserves, pickled vegetables, and other foods can be seen Ace-K figure. These applications not only improve the taste and quality of food but also provide consumers with more diversified healthy choices.4
Science Anecdotes
During the research and development of Acesulfame Potassium, many interesting stories and discoveries have emerged. For example, when scientists studied its sweetness mechanism, they found that it interacts in a unique way with sweetness receptors in the human taste system. This mode of action not only explains why it has such a high degree of sweetness but also provides valuable implications for the development of new sweeteners.
In addition, its widespread use has spawned many interesting business innovations. Some companies have taken advantage of its low-calorie properties to launch "sugar-free" or "low-sugar" product lines, attracting a large number of consumers who are pursuing a healthy diet. These products not only meet the taste needs of consumers but also help them reduce sugar intake and calorie intake, achieving a win-win situation of health and taste.5
Conclusion
With the increasing demand for a healthy diet and the continuous development of the food industry, Acesulfame Potassium will continue to play an important role as an excellent sweetener. In the future, we can expect the innovative application of it in more fields, as well as the continuous optimization and in-depth study of its performance by scientists.
References:
[1] NETTER K J. Alternative sweeteners[J]. Toxicology, 1988, 48 1: 1-117. DOI:10.1016/0300-483X(88)90067-4.[2] AHMAD ALI. Antiglycating potential of acesulfame potassium: an artificial sweetener.[J]. Applied Physiology, Nutrition, and Metabolism, 2017, 42 10. DOI:10.1139/apnm-2017-0119.
[3] KRISTINA I ROTHER. Pharmacokinetics of Sucralose and Acesulfame-Potassium in Breast Milk Following Ingestion of Diet Soda.[J]. ACS Applied Bio Materials, 2018. DOI:10.1097/MPG.0000000000001817.
[4] YOSHINORI HANAWA. Acesulfame potassium induces dysbiosis and intestinal injury with enhanced lymphocyte migration to intestinal mucosa[J]. Journal of Gastroenterology and Hepatology, 2021, 36 11: 2993-3252. DOI:10.1111/jgh.15654.
You may like
See also
Lastest Price from Acesulfame potassium manufacturers

US $0.00-0.00/kgs2025-06-19
- CAS:
- 55589-62-3
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons

US $0.00-0.00/kgs2025-06-18
- CAS:
- 55589-62-3
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons