Guanidine Thiocyanate: A Comprehensive Overview for Chemistry Professionals
Guanidine thiocyanate is a compound widely used in molecular biology and biochemistry research. As a chaotropic agent, it can disrupt cell and organelle membranes, promote cell lysis and organelle separation. Notably, it is an effective protein denaturant, surpassing guanidine hydrochloride in unfolding proteins. This property is very useful for purifying nucleic acids from crude cell lysates, and can effectively separate nucleic acids from denatured proteins.

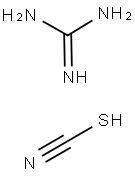
Figure 1 Characteristics of Guanidine thiocyanate
Uses
Guanidine thiocyanate is a powerful protein denaturant that plays an important role in the field of biotechnology. It can dissolve proteins and eliminate their secondary structures, decompose cell structures, and quickly separate nucleoproteins from nucleic acids. This property makes it widely used in the preservation of whole blood samples, the extraction of DNA and RNA, and cell lysis. Guanidine thiocyanate is of great value in the detection of new coronavirus nucleic acid. As the main component of nucleic acid lysate, it can quickly break up viruses and quickly separate nucleoproteins from nucleic acids. At the same time, it can inhibit the activity of released nucleases, ensure the integrity of the primary structure of nucleic acids, and improve the efficiency of nucleic acid extraction.
Applications
Guanidine thiocyanate is an effective chaotropic agent and denaturant that can effectively lyse cells. It has been used in a variety of molecular biology applications, including lysis of colorectal adenocarcinoma cell lines prior to RNA isolation, as a PCR inhibitor in triple mitochondrial DNA (mtDNA) qPCR assays, and to aid in DNA extraction from cerebrospinal fluid (CSF). Guanidine thiocyanate plays a key role in these processes because it is able to break down cell membranes and denature proteins, aiding in the release and purification of nucleic acids.
Biochemical/Physiological Actions
Guanidine thiocyanate is a denaturant commonly used for RNA isolation. It can be used as a storage buffer for whole blood samples. Guanidine thiocyanate inactivates nucleases, making it ideal for storing and freezing stool samples for DNA studies. Guanidine thiocyanate is used in combination with phenol-chloroform for RNA extraction.
Related articles And Qustion
See also
Lastest Price from Guanidine thiocyanate manufacturers

US $20.00/kg2025-04-27
- CAS:
- 593-84-0
- Min. Order:
- 100kg
- Purity:
- 99.5%min
- Supply Ability:
- 1 ton

US $100.00/KG2025-04-21
- CAS:
- 593-84-0
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON