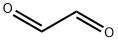Glyoxal: An Overview for Chemistry Professionals
Introduction
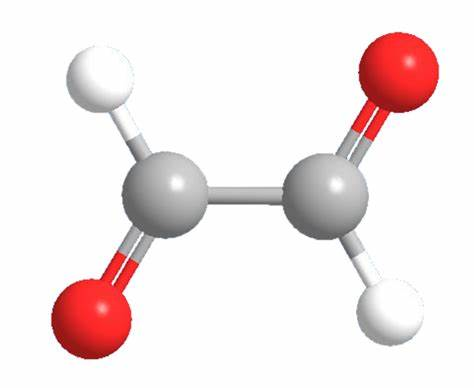
Glyoxal (OHC–CHO) is a dialdehyde that can be obtained from several natural sources, such as from the oxidation of lipids or as a by-product of biological processes. This compound is a physiological reactive α-oxoaldehyde metabolite produced by lipid peroxidation and autoxidation of glucose.

Uses
The low vapour tension of the glyoxal solution and its low toxicity are advantages of using Glyoxal instead of formaldehyde. The two adjacent carbonyl groups make Glyoxal highly reactive. Glyoxal is already used as a formaldehyde substitute in wood adhesive applications. Glyoxal was also used to prepare Glyoxal–phenolic pre-polymers in alkaline conditions to make composites reinforced with sisal fibers. Some molecular structures of the resins have been proposed based on NMR analyses. Regardless of the cure cycle used, the reinforcement of thermosets by 30% (w/w) sisal fibers improved the impact strength by one order of magnitude. Limiting the curing temperature to 150°C, the composites displayed good adhesion between the resin and the sisal fibers and excellent dynamic–mechanical properties. Furthermore, Glyoxal is one of the most important volatile organic compounds in the atmosphere and the tracer of the photochemical oxidation process of VOCs, which can effectively reflect the emission level and reactivity of atmospheric VOCs.
Toxicity
Glyoxal is a physiological metabolite formed by lipid peroxidation, ascorbate autoxidation, oxidative degradation of glucose, and degradation of glycated proteins. Glyoxal has been linked to oxidative stress and can cause several cellular damages, including covalent modification of amino and thiol groups of proteins to form advanced glycation end products. After incubation of isolated liver mitochondria with Glyoxal, disrupted electron transport chain, increased mitochondrial ROS formation, lipid peroxidation, mitochondrial membrane damage, GSH oxidation, and protein carbonylation ensued compared to the control group ( p < 0.05). Glyoxal toxicity in isolated rat liver mitochondria was dose-dependent. In conclusion, Glyoxal impaired the electron transport chain, which is the cause of increased ROS and MDA production, depletion of GSH, and disruption of MMP. Mitotoxicity of Glyoxal might be related to the pathomechanisms involved in diabetes and its complications.
References:
[1] M GOUDARZI M R H Kalantari. Glyoxal toxicity in isolated rat liver mitochondria.[J]. Human & Experimental Toxicology, 2018, 37 5. DOI:10.1177/0960327117715900.Related articles And Qustion
See also
Lastest Price from Glyoxal manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 107-22-2
- Min. Order:
- 1KG
- Purity:
- 40%
- Supply Ability:
- 20 tons

US $100.00/KG2025-04-21
- CAS:
- 107-22-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON