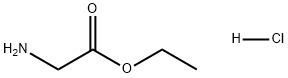
Glycine ethyl ester hydrochloride: Synthesis and Applications
Glycine ethyl ester hydrochloride (GEECl) is an effective additive to improve the device performance by regulating crystallization and passivating the defects at the GBs and interfaces. As a result, the GEECl-modified device delivered a PCE of 19.86 %, which is higher than the control device (18.11 %). Furthermore, more exploration can be done based on similar molecular structures, such as adjusting the size of the molecule to explore its distribution in the perovskite crystal or adding some functional groups to the molecule.

Synthesis of Glycine ethyl ester hydrochloride
Preparation of Glycine Alkyl Ester Hydrochlorides [GP 1] 1.2 equivalents of thionyl chloride are introduced into 0.6 ml of alcohol per mmol of glycine with ice cooling to -10 C. After removal of the ice bath, 1 equivalent of glycine is added in portions. The mixture is stirred for 2 hours while being refluxed. After cooling to room temperature, the excess alcohol and the thionyl chloride are removed in a rotary evaporator. The resultant white solid is combined twice with the alcohol and the latter is again removed in the rotary evaporator in order to remove any adhering thionyl chloride completely. B) glycine Ethyl Ester Hydrochloride (40) In accordance with GP 1, 1000 ml of ethanol are reacted with 130 g (1.732 mol) of glycine 39 and 247.3 g (2.08 mol) of thionyl chloride.After recrystallization from ethanol, a colorless, acicular solid is obtained, which is dried under a high vacuum. 1H-NMR spectrum (300 MHz, CD3OD): δ=4.30 (q, J=7.14, 2H, OCH2), 3.83 (s, 2H, H2CNH2), 1.32 (tr, J=7.14, 3H, CH3) ppm. 13C-NMR spectrum (75 MHz, CD3OD): δ=167.53 (C=O), 63.46 (OCH2), 41.09 (H2CNH2), 14.39 (CH3) ppm. All other analytical data are in line with literature values.[1]
Growth of glycine ethyl ester hydrochloride
The complex and the carboxylic acid are expected to throw light on the geometrical feature of bio-molecular interactions. The glycine molecule exists in the cationic form with a positively charged amino group and an unchanged carboxylic acid group . Recently literature reports shows that glycine combines with H2SO4, AgNO3, CaCl2, CaNO3, BaCl2. The reaction of the glycine ethyl ester and hydrochloride yields glycine ethyl ester hydrochloride.[2]
Scientists report the growth and the result of characterization studies of glycine ethyl ester hydrochloride by slow evaporation method. The grown crystals were characterized by various methods such as optical, XRD, Z-scan, dielectric method, mechanical and thermal methods and the results are analyzed and discussed for the first time. The fluorescence spectrum emits blue light at 488 nm. The bulk resistance and dc conductivity were determined by complex impedance spectroscopy. The dc conductivity (σdc) was obtained by 3.60×10−5 mho/m at room temperature in Glycine ethyl ester hydrochloride crystal. The dielectric studies reveal that the behavior of space-charge polarization of the material. The microhardness study shows that the Glycine ethyl ester hydrochloride crystal belongs to soft material category. The TG/DTA studies reveal that the Glycine ethyl ester hydrochloride crystal decomposes and is stable upto 174 °C. Finally, we concluded that the material may be utilized in the NLO device applications.
Crystal quality and defects at grain boundaries (GBs) or grain surfaces are two dominant factors in perovskite solar cells (PSCs) that determine device performance. Here, a novel amino acid salt named glycine ethyl ester hydrochloride (GEECl) is first introduced into perovskite to improve crystal quality and reduce GBs. Owing to fewer GBs and better vertically extended crystals, PSCs with GEECl exhibit improved carrier transport ability. The defects at GBs or grain surfaces can be well controlled by filling the MA+ vacancy with –NH3+ and the interaction between the carbonyl group (C═O) and uncoordinated Pb2+. As a result, the GEECl-modified devices based on MAPbI3 achieved a higher efficiency of 19.86 % with an FF close to 80 %. Furthermore, the GEE+ cations locate at GBs or grain surfaces protect the perovskite films against moisture, which enhanced the stability of PSCs. This work provides a promising route to further accelerate the development of PSCs.[3]
References
[1] GRUENENTHAL - US2003/236429, 2003, A1
[2] Venkatesan, G., & Pari, S. (2016). Growth of glycine ethyl ester hydrochloride and its characterizations. Physica B: Condensed Matter, 501, 26–33.
[3] Li, J., Li, B., Yang, G., Zheng, D., & Yu, J. (2023). Crystallization and defects regulation of efficient inverted perovskite solar cells via glycine ethyl ester hydrochloride. Applied Surface Science, 608, 155269.
See also
Lastest Price from Glycine ethyl ester hydrochloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 623-33-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00-0.00/kg2025-04-21
- CAS:
- 623-33-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+