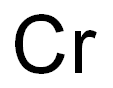General Properties of Chromium
Chromium [7440-47-3], atomic number 24 and relative atomic mass 51.9961(6), is the first element of group VIB(6) of Mendeleev’s periodic chart and was named after the Greek khroma, meaning color, due to the various colors of chromium species. Chromium is a steelgray, lustrous, brittle, and hard metal with a Mohs hardness of 8.5 that takes a high polish. Chromium, with a density of 7150 kg.m–3, is less dense than ferrous elements (i.e., Fe, Ni, Co, and Mn) with which it is often alloyed. Due to its high melting point of 1857°C, chromium is considered a refractory metal.

History
The history of chromium as a chemical element began in 1761, when Johann Gottlob Lehmann discovered an orange-red mineral at Beresof Mines in the Ural Mountains, called at that time Siberian red lead and today known as crocoite (PbCrO4). Following this discovery, the use of red lead as a pigment in the paint industry started immediately.
General Properties
Naturally occurring chromium is composed of four stable isotopes: 50Cr (4.35 at.%), 52Cr (83.789 at.%), 53Cr (9.50 at.%), and 54Cr (2.36 at.%). Chromium, like other refractory metals, exhibits a typical valve action (VA) property, that is, it forms an impervious passivating layer of chromium oxide upon exposure to any oxygenated media. This impervious and hard layer protects the metal from most corrosive chemicals and also provides an excellent oxidation resistance in air.
From a chemical point of view, the most common oxidation states of chromium are +2, +3, and +6, with trivalent chromium being the most stable as it is an essential element important in animal nutrition and hence not considered hazardous. Hexavalent chromium, which exists either as the chromate anion (CrO4 –) in alkaline media or as dichromate anion (Cr2O7 2–) in acidic media, is a powerful oxidant that displays acute and chronic toxicity properties such as carcinogenicity. Hence hexavalent chromium poses serious occupational and environmental hazards.
Chromium is resistant to hydrobromic and hydroiodic acids, aqua regia, phosphoric acid, and all organic acids (e.g., acetic, formic, oxalic).
Moreover, chromium metal is highly resistant to alkalis such as ammonia, concentrated solutions of sodium and potassium hydroxides, and sodium carbonate. At high temperatures chromium metal reacts with nitrogen, oxygen, carbon, and halogens.
You may like
Related articles And Qustion
See also
Lastest Price from Chromium manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7440-47-3
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $12.50-10.50/kg2025-01-16
- CAS:
- 7440-47-3
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 1000ton/Year