Ganciclovir: Antimicrobial Activity and Pharmacokinetics
Introduction
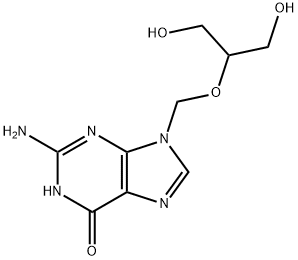
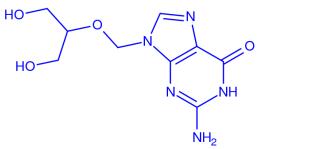
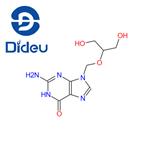
Ganciclovir is a nucleoside analogue that differs from acyclovir by having an extra hydroxymethyl group on the acyclic side chain. Ganciclovir's greatest in vitro activity is against CMV, although it is also as active as acyclovir against HSV-1 and HSV-2 and almost as active against VZV.
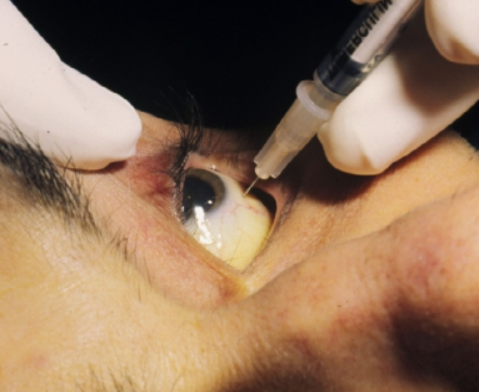
Antimicrobial Activity
Ganciclovir is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of cytomegalovirus retinitis (CMV retinitis) in adults who are immunocompromised, including individuals with AIDS. Ganciclovir is a synthetic guanine derivative that specifically inhibits viral DNA synthesis by inhibiting viral DNA polymerase resulting in termination of viral DNA elongation. Ganciclovir is active against CMV and, to a lesser extent, HSV.
Pharmacokinetics
Ganciclovir is phosphorylated by viral protein kinases to ganciclovir triphosphate, the active metabolite that persists for days in CMV-infected cells. To a lesser degree, normal, uninfected cells can generate monophosphorylated ganciclovir, thus possibly explaining its cytotoxic side effects. Ganciclovir exhibits low protein binding. CSF penetration is 24% to 70% of the respective plasma concentrations. Renal excretion of unchanged drugs occurs by glomerular filtration and active tubular secretion. Half-life in adults is 3.5 hours. Dose adjustment is advised in renal insufficiency. Ganciclovir exhibits linear kinetics with dose escalation.
Single-dose, intravenous ganciclovir PK has been evaluated in 27 neonates less than 50 days old. After a 4 mg/kg or 6 mg/kg intravenous dose, the peak concentration was 5.5 and 7.0 μg/mL, respectively; systemic clearance was 3.14 and 3.56 mL/kg/min, respectively; and the half-life was 2.4 hours for both doses. Clearance was less than the 4.7 mL/kg/min reported in children, but the half-life was similar; neonates had high interpatient variability. Population PK analysis of these newborns showed that ganciclovir clearance was associated with body weight and creatinine clearance. The mean population clearance was 0.4 L/hr, and the volume of distribution was 1.73 L. A recent population PK analysis in young infants receiving intravenous ganciclovir or oral valganciclovir showed similar results, namely a mean clearance of 0.32 L/hr and volume of distribution of 1.78 L. Because ganciclovir is eliminated through the kidney, we would expect a delay in drug clearance during the first week of life and in extremely premature infants.
Safety
The Ganciclovir label contains a black box warning for the risk of granulocytopenia, anaemia, and thrombocytopenia, and the drug should be avoided in patients with cytopenia or a history of cytopenia. Dose reduction is also indicated in renal impairment. Additional reported adverse events include fever, anorexia, and vomiting. Ganciclovir has important drug interactions: it should not be combined with imipenem-cilastin due to concern for seizures, and caution is required when combined with other nucleoside analogues, cyclosporine, and amphotericin B due to potential additive toxicity.
In a placebo-controlled trial of ganciclovir in infants, neutropenia occurred in 63% of infants receiving ganciclovir compared with 21% in the control infants. Adverse events in infants participating in a phase 2 study of 8 versus 12 mg/kg/day included retinal detachment, neutropenia, and a moderate increase in creatinine and liver enzymes. It remains unclear if these adverse events were attributed to either progression of CMV disease, adverse drug effects, or both. Periodic monitoring of blood counts, serum creatinine, and liver function is recommended, and hematologic manifestations are typically transient.
You may like
Related articles And Qustion
Lastest Price from Ganciclovir manufacturers
US $0.00/kg2025-11-25
- CAS:
- 82410-32-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $0.00/kg2025-09-30
- CAS:
- 82410-32-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg