Furazolidone: History, Mechanism of Action and Adverse Effects
General Description
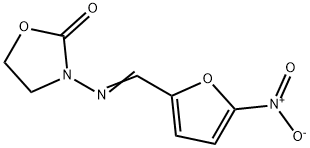
Furazolidone is a synthetic nitrofuran antimicrobial agent initially used for treating gastrointestinal infections and peptic ulcers before the identification of Helicobacter pylori as a primary cause. It functions by disrupting bacterial metabolism and DNA synthesis through its action as a monoamine oxidase inhibitor. Although resistance rates to furazolidone vary by region, they remain low in China, while some increases have been noted elsewhere. Common adverse effects include nausea, vomiting, and dizziness, with serious effects occurring rarely. Despite concerns over its potential carcinogenicity, no definitive evidence supports this in humans, emphasizing the importance of monitoring and patient education during treatment.

Figure 1. Furazolidone
History
Furazolidone, a synthetic nitrofuran antimicrobial agent, has traditionally been used primarily for the treatment of giardiasis and gastrointestinal (GI) infections. Prior to the identification of H. pylori, physicians in China had been using furazolidone to treat peptic ulcers for years, which achieved good clinical efficacy. Subsequently, peptic ulcers were found to be primarily caused by H. pylori infection, leading to the prescription of furazolidone as the treatment. Subsequently, various regimens combining antibiotics with acid suppressants and/or bismuth have been applied to eliminate H. pylori. With increasing H. pylori resistance to metronidazole, the eradication rate of standard triple therapy has declined to an unacceptable level. As a result, quadruple therapy containing furazolidone, as a substitute for metronidazole, exhibits promising effectiveness.
In the 1990s, animal experiments showed that furazolidone had potential mutagenicity, genotoxicity, and carcinogenicity. Although the researchers suggested that the relationship between bacterial mutagenicity and animal genotoxicity of furazolidone might be fortuitous, the United States Food and Drug Administration (FDA) implemented a prohibition of all nitrofuran drugs, while the European Medicines Agency also restricted their use. Currently, furazolidone remains unavailable in the United States and Europe; however, it is still licensed for clinical use in several countries including China, Iran, Mexico, and Pakistan. In 2018, the China National Medical Products Administration (NMPA) mandated the modification of the indications of furazolidone use to solely encompass "difficult-toeradicate H. pylori infection", thereby emphasizing cautious administration of this drug. In 2019, the NMPA issued a directive to cease the production, sale, and utilization of compound preparations containing furazolidone in China. Increasing restrictions in recent years have led to a rapid decline in the use of furazolidone-containing regimens.1
Mechanism of Action
Furazolidone is a broad-spectrum nitrofuran antimicrobial agent with high concentrations in the GI tract after oral administration. Both in vivo and in vitro studies have confirmed that furazolidone has potent activity against H. pylori.
As a monoamine oxidase (MAO) inhibitor, furazolidone has strong antioxidative activity but can be reduced by a variety of enzymes. It interferes with bacterial oxidoreductases, inhibits acetyl-coenzyme A and other enzymes, and inhibits the synthesis of ribosomal proteins and other large molecular proteins, resulted in bacterial metabolic disorder and DNA damage.1
H. Pylori Resistance to Furazolidone
The effectiveness of furazolidone against H. pylori is notable, with reported resistance rates varying across geographical regions. In China, the rate of H. pylori resistance to furazolidone is relatively low, estimated at around 0% to 4%. However, resistance rates have seen increases in other regions, such as Iran, where resistance jumped from 21.6% to 27.2% in a ten-year span. This contrasts with reports from Asia and South America, showing rates of 23% and 0%, respectively. Interestingly, mutations in the porD and oorD genes of H. pylori have been implicated in the development of resistance to furazolidone. Importantly, furazolidone does not produce cross-resistance with other antibiotics, resulting in a low incidence of secondary drug resistance in treated patients. This unique characteristic, along with its multifaceted action, underscores the importance of monitoring resistance patterns to maintain the efficacy of furazolidone in clinical settings. 1
Adverse Effects
Furazolidone, a nitrofuran antimicrobial agent, is associated with various adverse effects, although these are generally not significantly higher than those linked to other antibiotic therapies. Common adverse effects of furazolidone include nausea, vomiting, diarrhea, dizziness, headache, drug fever, rash, and orthostatic hypotension. Less frequently, serious conditions such as hemolytic anemia, jaundice, and polyneuritis can occur. The likelihood of adverse effects increases with higher dosages, prolonged treatment durations, and the concurrent use of bismuth. A typical safe daily dose of furazolidone is around 200 milligrams, recommended for a treatment period of 10 to 14 days for effective eradication of Helicobacter pylori. Although concerns regarding the potential carcinogenic effects of furazolidone exist, no definitive evidence confirms such risks in humans. Moreover, furazolidone acts as a monoamine oxidase inhibitor, necessitating avoidance of certain foods and alcohol during treatment to prevent harmful interactions. Regular follow-up and patient education about furazolidone's possible adverse reactions are essential for safe therapy. 2
References:
[1] YING YING HAN. Application of furazolidone in Helicobacter pylori infection eradication[J]. Journal of Digestive Diseases, 2024, 25 3: 147-211. DOI:10.1111/1751-2980.13265.[2] MARJAN MOHAMMADI. Furazolidone, an Underutilized Drug for H. pylori Eradication: Lessons from Iran.[J]. Digestive Diseases and Sciences, 2017, 62 8. DOI:10.1007/s10620-017-4628-5.
You may like
Related articles And Qustion
Lastest Price from Furazolidone manufacturers

US $0.00-0.00/kg2025-11-19
- CAS:
- 67-45-8
- Min. Order:
- 25kg
- Purity:
- 97.0%~103.0%; BP2015
- Supply Ability:
- 50tons/month

US $0.00/kg2025-04-25
- CAS:
- 67-45-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000kg